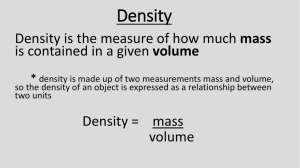Density
advertisement

Bellringer 9-14-09 Calculate the following 9.0 x 1023 - 9.0 x 1021 Mass vs volume Thought question Which is heavier a pound of lead or a pound of feathers? Most people say lead… They are thinking about if you have the same size piece of lead and feathers obviously the lead will be heavier. However, a pound of lead is much smaller in size than a pound of feathers Why? The answer is density Lead atoms are packed tightly together Feathers are not Density is… The ratio of the mass of an object to its volume Density = mass/volume A 10.0 cm3 piece of lead has a mass of 114 g. How would you find density? Density of lead is 11.4 g/cm3 Density As mass increases, density increases As volume increases, density decreases Depends only on substance not on size A block of maple wood with a volume of 405 cubic centimeters and a density of 0.67 g/cm3 is sawed in half. The density of the two smaller blocks is now -A one-fourth the original density B one-half the original density C two times the original density D the same as the original density Densities of common materials Can you find any themes? Water is 1 g/cm3 Density Trends Solids usually most dense Gases usually least dense Exception is water Ice is less dense than liquid water Water expands as it freezes and creates air pockets Volume increases and mass remains constant, therefore density decreases Floating is related to density A substance that is less dense will float in a substance that is more dense Example… Helium density 0.166 g/L Air density 1.2 g/L Water density 1 g/cm3 Oil density 0.922 g/cm3 Ice density 0.917 g/cm3 Steel density approx. 8 g/cm3 What about ships? Calculations What is the density of a piece of wood that has a mass of 25.0 grams and a volume of 29.4 cm3? D=m/v m=25.0 g v=29.4 cm3 25.0 / 29.4 D = 0.850 g/cm3 A piece of wood that measures 3.0 cm by 6.0 cm by 4.0 cm has a mass of 80.0 grams. What is the density of the wood? Would the piece of wood float in water? D = 1.1 g/cm3 The density of aluminum is 2.70 g/mL. If the mass of a piece of aluminum is 244 grams, what is the volume of the aluminum? Use the triangle Cover up what you are looking for It will tell you math to do V=m/D V = 90.4 mL Water displacement Silly putty with a mass of 8.0 g is placed in a graduated cylinder with an initial water level of 25 ml After placing silly putty into graduated cylinder the water level rose to 29 ml. What is the density of the silly putty? 29 ml – 25 ml = 4 ml D = 8 g / 4 ml D = 2 g/ml