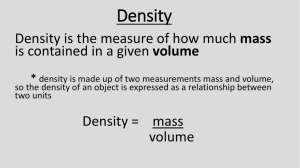A hypothesis is best described as
advertisement

Chemistry Study Guide: Test #1 A hypothesis is best described as: An educated guess If …….then…….. The scientific method is: A systematic approach to answer a question - testable A scientific theory is best described as: An explanation supported by many experiments The dependent variable is best described as: The one that changes; located on the Y axis In an experiment, the independent variable is the one that: “I” can control; located on the X axis The best way to find the volume of a marker is called: Water displacement The formula for density is: D = m/v In determining how many significant figures a number has, you must first look for a decimal If a decimal is present, then you start counting from the front (left) If a decimal is absent, then you start counting from the back (right) Pacific Atlantic The first rule of lab safety is: Tell the teacher Volume is measured in mL or cm3, which are the same Density is written in which units? g/mL or g/cm3 When measuring irregular objects (such as cylinders, marbles, rocks) you should use water displacement as the method of finding volume. You can only use the L*W*H method of finding volume when objects are considered regular in shape or easily measured with a ruler. Metric Measurements Kilo 1000 Hecto 100 Deca 10 m L g deci centi milli 10 100 1000 Kids have dropped over dead converting metric Which prefix means 1,000? Kilo Which prefix means 1/100? centi Which prefix means 1/1000? milli Read the following graduated cylinders. Make sure to include (mL) in your answer 52.9 mL 6.64 mL 20.6 mL Descriptor Qualitative or Quantitative Red Qualitative 17 centimeters Quantitative 46 desks Quantitative Rusty Qualitative 48 marbles Quantitative 152 milliliters Quantitative Smelly Qualitative Calculate the densities of the following objects. Remember to place units after each number. Object A: Object B: Length = 6 cm Width = 3 cm Height = 1 cm Mass = 36 g Length = 10 cm Width = 5 cm Height = 2 cm Mass = 300 g Volume = 18 cm3 Density = 2 g/cm3 Volume = 100 cm3 Density = 3 g/cm3 Object C: Use the water displacement method to determine the density of Object C (silly putty) Initial water level in graduated cylinder = 25 mL Water level after placing silly putty into graduated cylinder = 29 mL Mass of silly putty = 8g Volume = 4 mL Density = 2 /mL Which of the following materials will float on water (density = 1.00 g/cm3)? Corn oil = .93 g/cm3 -- float Glycerine = 1.26 g/cm3 -- sink Corn syrup = 1.38 g/cm3 -- sink Wood = .85 g/cm3 -- float Steel = 7.81 g/cm3 -- sink Rubber = 1.34 g/cm3 -- sink Ice = .92 g/cm3 -- float Water = 1.00 g/cm3 -- neither Round the following to 3 significant figures 124.78 1.001 56.0 78.490 175.689 125 1.00 56.0 78.5 176 Complete the following calculations be sure to use the proper number of significant figures 51.8 kg + 65.23 kg = 117 Kg 1 m3 16 m x 31 m x 0.003 m = 1.488 3.45 m ∕ 2.1 s = 1.6 m/s 45.21 x 3.0 = 136.63 12.01 g - 6.0 g = 6.01 140____ 6.0 g Describe each of the following as: accurate – precise – both - none None BOTH Precise None