File
advertisement

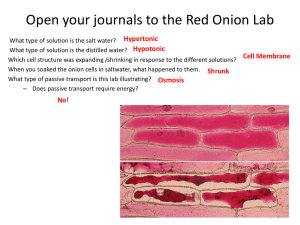
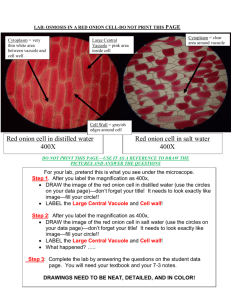
Vinita Patel Nola Kane 10/15/12 - 10/16/12 Period 4/5 Osmosis of Tap, Salt and Distilled Water in Red Onion Cells Introduction: The three types of cell transport are passive, active and bulk. Passive transport requires no energy and goes down or with the concentrated gradient. In addition, it includes simple diffusion, facilitated diffusion and osmosis. Active transport requires energy and goes up or against the concentrated gradient. Bulk transport requires energy and it uses the cell membrane for endocytosis and exocytosis. The red onion lab is dealing with osmosis. There are three types of osmosis conditions isotonic, hypertonic and hypotonic. Isotonic is when the water inside the cell is the same as outside the cell. Then, hypertonic is when there is more water inside the cell then outside, so then the water moves out of the cell. Turgor can happen in plant cells if there is a lot of water in the cell. Last, hypotonic is when there is more water outside of the cell than inside, so it moves inside the cell. It can also cause plasmolysis. Next, there are different types of vacuoles in one of them is the central vacuole. The central vacuole stores water in plant cells. Another one is the contractile vacuole, they are in protists and they pump out the excess water that is in the cell. The organelles that are pertinent to this lab are the nucleus, cell membrane, cell wall and the central vacuole. The hypothesis for this experiment is that in the distilled water the red onion cell will experience turgor because all the water is going into the cell because it is very hypotonic. The salt water will cause the cell to be hypertonic because the salt is the solute and causes there to be less water. So the cell with undergo plasmolysis. Last, the tap water will cause the onion cell to be hypotonic because it is just water like there is no solute, so the water will go into the cell and make the cell full and cause some turgor. Objective: To determine the effects of tap, salt and distilled water in red onion cells. Methods: Day 1The materials that were used in this experiment were the red onion epidermis, tap water with dropper, glass slide, salt water with dropper, cover slip, distilled water with dropper, paper towel, jar lids (to draw circles for drawings) and a compound light microscope. First, make a wet mount slide (using tap water) of a piece of red onion epidermis. Use one drop of the tap water on one side of the cell. Be sure to start with skimming the slide and getting it focused. Second, examine the slide under low power (100x) on the compound light microscope. Then, choose a cell toward the edge of the slide and place it in the very center of the field of view. Next, switch to high power (400x) and make a neat drawing of that cell. Be sure to label the cell membrane, the cell wall, and the central vacuole on that cell. In addition, label the drawing Fig 1.Besides the drawing of the cell write the general appearance of the cell. Then, use a small piece of paper towel the straight side of it to soak the tap water from the slide. Do this till most of the water is gone and then add the salt water from the opposite side from the tap water was on. Follow what was done for the tap water including the drawing and observations but, label the drawing Fig 2. Last, clean up all material properly. Day 2 First, make a wet mount slide (using that last type of water which is distilled) of a piece of red onion epidermis. Use one drop of the distilled water on the slide. Start with skimming the cells then, change to 100x on the compound microscope and choose a cell toward the edge of the slide. Make sure it is placed in the center field of view. Next, turn to 400x and follow what was done for the tap and salt water including the drawing and observations but, label the drawing Fig 3. Last, clean up all material properly. Data/Results: Analysis: The data reported for red onion cells in tap water does not support the hypothesis. The hypothesis was wrong because tap water with the red onion cells does not have a hypotonic osmotic condition. The red onion cells in the tap water have the type of osmotic condition of isotonic. There was nothing wrong with the red onion cells. There was no turgor or plasmolysis just normal looking full and complete. The direction of osmosis was in and out at the same rate. Furthermore, the cell situation created by the tap water was dynamic equilibrium. The hypothesis was right for the salt water in the red onion cells. In addition, the red onion cells in salt water have the osmotic condition called hypertonic. That is why the cell was all shriveled up. The cell wall was smaller than the cell membrane. Also, the direction of osmosis was out of the cell. Last, the cell situation created by the salt water was plasmolysis. For the distilled water with the red onion cells, the hypothesis was right. It was indeed very hypertonic. The cell looked swelled and actually a little bigger in size. The direction of osmosis was into the cell. Finally, the cell situation that was created by the distilled water was turgor. All three solutions added to the red onion cell each made a different osmotic condition. Also, all the red onion cells still had the same color of purplish, reddish, pinkish. The cell membranes did not change size in all three solutions, only the cell wall did. The red onion cells looked physically different through the microscope. With the salt and isotonic onion cells the nucleus was able to be found. The nucleus was not found with the distilled water. The independent variable was only the type of water that was with the onion cells. Conclusion: 1. The term that is used to describe red blood cells swelling and bursting is cytolysis. This does not happen to red onion cells because it is a plant. Plant cells have cell walls to prevent them from bursting. They hold in the water and they undergo turgor. 2. Grocery store owners spray fresh fruits and vegetables with water because they don’t want the food to look bad and not fresh. Furthermore, to retain color, freshness or crispness, and cleanliness. In addition, they do not want the plants to shrivel up and cause a hypertonic situation. 3. Salted roads cause roadside plants to die. The salt causes plant surroundings to lesson in concentration making the plant release all its water and die. Plasmolysis happens to the plants. 4. I think a shipwrecked crew would die if they drank their own urine because their urine has salt in it. In addition, other wastes that your body doesn’t need. Salt will make your cells hypertonic and my you even more dehydrated, causing them to die. 5. The fresh straw berries sprinkled in sugar turn to juice after a few minutes because osmosis happened. There is more water inside the strawberry then outside of it. So the water inside the strawberry will begin to leave the cells until there is equal water on both sides of the cell membrane. That is what makes the strawberries juicy. This osmotic condition is called hypertonic.