Lipids are non
advertisement

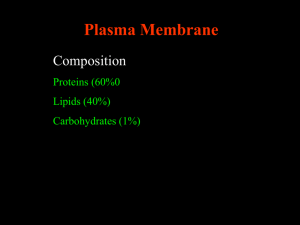
Lipids & Membranes Lipids Lipids are non-polar (hydrophobic) compounds, soluble in organic solvents. Most membrane lipids are amphipathic, having a non-polar end and a polar end. Fatty acids, the simplest lipids, consist of a hydrocarbon chain with a carboxylic acid at one end. an 16-C fatty acid: CH3(CH2)14-COONon-polar polar O Abbreviated notation a C 3 1 O for a 16-C fatty acid 4 2 with one cis double bond between carbons fatty acid with a cis-9 9 &10 is 16:1 cis 9. double bond Some examples: 14:0 myristic acid 16:0 palmitic acid 18:0 stearic acid 18:1 cis9 oleic acid 18:2 cis9,12 linoleic acid 18:3 cis 9,12,15 a-linonenic acid 20:4 cis 5,8,11,14 arachidonic acid 20:5 cis 5,8,11,14,17 eicosapentaenoic acid Double bonds in fatty acids are usually have the cis configuration. 4 a 3 2 O C 1 O fatty acid with a cis-9 double bond Most naturally occurring fatty acids have an even number of carbon atoms. There is free rotation about C-C bonds in a fatty acid, except at a double bond. Each cis double bond causes a kink in the chain. Rotation about other C-C bonds would permit a more linear structure than shown above, but with a kink. Glycerophospholipids Glycerophospholipids (phosphoglycerides), are common constituents of cellular membranes. They have a glycerol backbone. Hydroxyls at C1 & C2 are esterified to fatty acids. An ester forms when a hydroxyl reacts with a carboxylic acid, with loss of H2O. CH2OH H C OH CH2OH glycerol Formation of an ester: O R'OH + HO-C-R" O R'-O-C-R'' + H2O Phosphatidate O O R1 C H2C O O CH H2C C R2 O O phosphatidate P O O The simplest glycerophospholipid is phosphatidate, in which fatty acids are esterified to hydroxyls on C1 & C2, while the C3 hydroxyl is esterified to Pi. O O R1 C H2C O O CH H2C C R2 O O P O X O glycerophospholipid In most glycerophospholipids (phosphoglycerides), Pi is in turn esterified to OH of a polar head group (X), e.g.: serine, choline, ethanolamine, glycerol, or inositol. The 2 fatty acids tend to be non-identical. They may differ in length and/or the presence/absence of double bonds. O O R1 C H2C O O CH H2C C R2 O O P O CH3 O CH2 CH2 + N CH3 CH3 phosphatidylcholine Phosphatidylcholine, with choline as polar head group, is an example of a glycerophospholipid. It is a common membrane lipid. O O R1 C H2 C O O CH H2 C C R2 O O P O O H OH OH H OH phosphatidylinositol OH H H H H OH Phosphatidylinositol, with inositol as polar head group, is another example of a glycerophospholipid. In addition to being a membrane lipid, phosphatidyl inositol has roles in cell signaling. Glycerophospholipid Each glycerophospholipid includes a polar region: glycerol, carbonyl of fatty acids, Pi, & polar head group (X) 2 non-polar hydrocarbon tails of fatty acids (R1, R2). Such an amphipathic lipid is often represented as at right. O O R1 C H2C O O C CH H2C R2 O O P O O glycerophospholipid polar "kink" due to double bond non-polar X OH Sphingolipids are derivatives of the lipid sphingosine, which has a long hydrocarbon tail, and a polar domain that includes an amino group. H2C OH H C CH H3N+ CH HC (CH2 )12 OH H2C O OH H C CH NH CH C R ceramide HC (CH2 )12 CH3 sphingosine CH3 The amino group of sphingosine can form an amide bond with a fatty acid carboxyl to yield a ceramide. Ceramides usually include a polar head group, esterified to the terminal OH of the sphingosine. CH3 H3C + N O H2 C H2 C O CH3 Sphingomyelin, a ceramide with a phosphocholine or phosphethanolamine head group, is a common constituent of plasma membranes P O O phosphocholine H2C sphingosine O fatty acid OH H C CH NH CH C R Sphingomyelin Sphingomelin, with a phosphocholine head group, is similar in size and shape to the glycerophospholipid phosphatidyl choline. HC (CH2 )12 CH3 Polar head groups of sphingolipids (ceramides): Sphingolipid Polar head group sphingomyelin phosphocholine or phosphoethanolamine cerebroside a monosaccharide such as glucose or galactose ganglioside a complex oligosaccharide, including the acidic sugar sialic acid Gangliosides, which have complex oligosaccharide head groups, are often found in the outer leaflet of the plasma membrane bilayer, with sugar chains extending out from the cell surface. Cholesterol HO Cholesterol Cholesterol has a rigid ring system and a short branched hydrocarbon tail. It is largely hydrophobic, but has one polar group, a hydroxyl, making it amphipathic. Cholesterol is found in membranes, and is the precursor for synthesis of steroid hormones and vitamin D. Monolayer at air/water interface Bilayer Spherical Micelle Amphipathic lipids form complexes in which polar regions are in contact with water and hydrophobic regions away from water. Depending on the lipid and its concentration, possible molecular arrangements include: A monolayer at an air/water interface. Various micelle structures. A spherical micelle is a stable configuration for amphipathic lipids with a conical shape, such as fatty acids. A bilayer. This is the most stable configuration for amphipathic lipids with a cyllindrical shape, e.g., phospholipids. Membrane fluidity: The interior of a lipid bilayer is normally highly fluid. liquid crystal crystal In the liquid crystal state, hydrocarbon chains of phospholipids are disordered and in constant motion. At low temperature, bilayer phospholipids may undergo transition to a crystalline state in which fatty acid tails are fully extended, packing is highly ordered, & van der Waals interactions are maximal. Kinks in fatty acid chains, due to cis double bonds, interfere with packing of lipids in the crystalline state. Thus double bonds inhibit transition to the crystalline state, & lower the phase transition temperature. Cholesterol inserts into bilayer membranes with its OH adjacent to polar phospholipid head-groups, and its hydrophobic ring system adjacent to fatty acid chains of phospholipids. Being slightly shorter than a phospholipid, cholesterol extends not quite to the center of the bilayer. Cholesterol in membrane Cholesterol inhibits transition from liquid crystal to crystalline state, because the rigid cholesterol ring interferes with close packing of phospholipid fatty acid tails. However the rigid cholesterol makes the membrane somewhat less fluid. There are 2 strategies by which phase changes of membrane lipids are avoided: Cholesterol is abundant in membranes, such as plasma membranes, that include many lipids with long-chain saturated fatty acids. Cholesterol blocks transition to the crystalline state. Membranes that lack cholesterol, e.g., mitochondrial inner membrane, consist mainly of phospholipids whose fatty acids include one or more double bonds. The double bonds lower the melting point to below physiological temperature. Cellular membranes contain a mixture of lipids. The fatty acid moiety of sphingolipids tends to be saturated. Glycerophospholipids often include at least one fatty acid that is kinked due to one or more double bonds. Sphingolipids tend to separate into sphingolipid-rich membrane microdomains, called lipid rafts. This has been attributed to the close packing of sphingolipids, with their fully saturated hydrocarbon tails. peripheral Membrane proteins peripheral integral having a lipid anchor lipid anchor lipid bilayer integral Membrane Proteins Peripheral proteins are on the membrane surface. They are water-soluble, with mostly hydrophilic surfaces. Many peripheral proteins adhere to exposed domains of integral proteins, e.g., by ionic & H-bond interactions. They often can be dislodged by high salt concentrations, change of pH, and/or chelators that bind divalent cations. Some cytosolic proteins temporarily bind to membranes, via domains that recognize and bind to particular lipids that transiently exist in the membrane. For example: Pleckstrin homology domains (PH) bind phosphorylated derivatives of phosphatidylinositol. A PH domain has a unique sandwich structure (2 orthogonal sheets capped at one end by an amphipathic a helix). Many signal proteins include PH domains, that promote their binding to the cytosolic surface of the plasma membrane, where they cooperate in signal cascades. Kinases that transfer Pi to the inositol moiety of phosphatidylinositol, creating ligands for PH domains, are themselves regulated by signal pathways. O O R1 C H2C O O C CH H2C R2 O O P O O phosphatidylinositol3-phosphate OH 2 H H 1 6 OH H 2 H OPO 3 3 H 4 OH 5 H OH FYVE domains bind to a particular phosphatidylinositol derivative, phosphatidylinositol-3-phosphate. FYVE domains include specific structural motifs in which zinc ions are bound by Cys & His residues. O O R1 C H2C O O C R2 CH H2C OH diacylglycerol C1 domains recognize and bind to diacylglycerol, which is generated by signal-activated cleavage of a phosphorylated derivative of phosphatidylinositol. C1 domains are named for a domain in an enzyme Protein Kinase C, that is activated by diacylglycerol. C1 domains are similar in structure to FYVE domains. peripheral Integral proteins have domains that extend into the hydrocarbon core of the membrane. Often they span the bilayer. lipid anchor lipid bilayer integral Membrane Proteins Structural & spectroscopic data indicate that a layer of relatively immobilized lipid surrounds the transmembrane portion of an integral protein. Hydrocarbon tails of lipids at the protein-lipid interface assume conformations that allow them to fill in surface irregularities of protein domains exposed to the bilayer. Integral proteins can be removed from membranes only by treatment with detergents, amphipathic molecules that break up the lipid bilayer. membrane detergent solubilization polar non-polar Protein with bound detergent Hydrophobic domains of detergents substitute for lipids in coating hydrophobic surfaces of integral proteins. This allows the proteins to dissolve in water. Without detergents, purified integral proteins tend to aggregate, as their hydrophobic surfaces come together, to minimize contact with water. CH3 CH3 H3C C CH CH2 CH2 C CH3 CH CH2 CH2 C CH CH2 S Protein farnesyl residue linked to protein via cysteine S Some proteins have a covalently attached lipid anchor, that inserts into the bilayer. Examples: fatty acid (myristic or palmitic acid) isoprenoid (e.g., farnesyl) GPI (glycosylphosphatidylinositol, a glycolipid). Such an anchor may allow reversible membrane association. Release may occur via: a conformational change that causes the attached lipid to be retracted into (buried within) the protein, or hydrolytic cleavage of the lipid (GPI). Lateral Mobility The Fluid Mosaic Model of membrane structure emphasizes the highly fluid character of the bilayer core and the ability of integral proteins and lipids to rapidly diffuse within the plane of the membrane. Lateral mobility of a membrane lipid is depicted above. Lateral diffusion of membrane lipids and proteins (within the plane of the membrane) is assayed by FRAP: Fluorescence Recovery After Photobleaching. FRAP technique: A membrane lipid or protein is tagged with a fluorescent dye. Proteins may be labeled with fluorescent antibodies. A laser bleaches (destroys fluorescence of) label in a region of membrane. Fluorescence recovers as undamaged label diffuses into the region. Generally lipids diffuse faster than proteins, which are larger. Protein diffusion may be constrained by proteinprotein interactions, e.g. association with cytoskeleton. Flip-flop of lipids (from one half of a bilayer to the other) is very slow. Flip-flop would require the polar head group of a lipid to traverse the hydrophobic membrane core. Flip Flop Flippase enzymes catalyze flip-flop in membranes where lipid synthesis occurs. Otherwise, flip-flop of lipids is rare. The 2 leaflets of a bilayer tend to differ in lipid composition. Flip-flop of integral proteins does not occur. All copies of a given type of integral protein have the same orientation relative to the 2 sides of the bilayer membrane. Transmembrane topology Transmembrane topology of membrane proteins is studied with membrane-impermeant probes, added on one side of a membrane: Protease enzymes (Degradation would indicate surface exposure of a protein segment.) Monoclonal antibodies raised to peptides equivalent to a segment of the protein sequence (Binding would indicate surface exposure of that protein segment.) Enzymes that attach labels to particular amino acids or sugars (Labeling would indicated surface exposure.) Membrane protein structure: Integral proteins are difficult to crystallize. Fewer integral proteins than soluble proteins have had their structure solved at atomic resolution. The most common structural motif in integral proteins is a membrane-spanning a-helix, consisting largely of hydrophobic amino acids. R-groups of a transmembrane a-helix would contact the hydrophobic membrane core. Aliphatic residues predominate in lipid-exposed protein domains toward the middle of the bilayer. Tyr & Trp are common closer to the membrane surface. The polar character of the Trp amide & the Tyr OH, in addition to their hydrophobic ring structures, may make them ideally suited for localization at the polar/apolar interface. Lys & Arg are often found in transmembrane segments, just outside the partly polar aromatics. Their positively charged groups, at the ends of aliphatic side chains, may extend outward to interact with polar lipid head groups. membrane Cytochrome oxidase dimer (PDB file 1OCC) An example of an integral protein whose intra-membrane domains consist mainly of transmembrane a-helices is cytochrome oxidase. Explore with Chime the a-helix colored green at the far left in this view. Porin -barrel A family of bacterial outer envelope channel proteins called porins have instead barrel structures. Porin Monomer At right is shown one channel of a trimeric channel complex. The barrel consists of sheet rolled up to form a cylindrical pore. PDB 1AOS polar R group non-polar R group In a -sheet, amino acid R-groups alternately point above & below the sheet. Much of porin primary structure consists of alternating polar & non-polar amino acids. Polar residues line the aqueous lumen of the channel. Non-polar residues are in contact with membrane lipids. Explore an example of a bacterial porin with Chime. Hydropathy plots A 20-amino acid a-helix just spans a lipid bilayer. Hydropathy plots are used to search for 20-amino acid stretches of hydrophobic amino acids in a protein for which a crystal structure is not available. Putative hydrophobic transmembrane a-helices have been identified this way in many membrane proteins. Protein topology studies are used to test the predicted transmembrane localization of protein domains. Hydropathy plots alone are not conclusive. Simplified helical wheel diagram of four a-helices lining the lumen of an ion channel. A “helical wheel” looks down the axis of an a-helix, projecting sidechains onto a plane. Polar amino acid R-group Non-polar amino acid R-group An a-helix lining a water-filled channel might have polar amino acid R-groups facing the lumen, & non-polar Rgroups facing lipids or other hydrophobic a-helices. Such mixed polarity would prevent detection by a hydropathy plot.