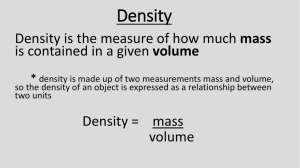13.5 g/cm 3
advertisement

•Metric System •Prefixes •Conversions •Scientific Notation •Writing •Calculating •Significant Figures •Definition •Counting •Calculating •Dimensional Analysis • AKA: International System (SI) • 1960: international agreement set up to use this system of units • Our “English system” is used within our boundaries, but we use the metric system in international trade. • 1999: NASA $125 million dollar mistake • Graphic organizer for prefixes – Mnemonic device: The good man King Henry died by drinking chocolate milk Monday night, poor fellow. – Prefixes – Powers of Ten – Place holders Oops! – Conversions • Temperature – Kelvin & Celsius As you come in, The Materials: 1. Put any email slips on the front desk. 2. Tarvin Consulting Group supplies 3. Pen/pencil and paper for a few notes The Plan: 1. Any questions about Element Quiz scheduled for Friday? 2. Work on Tarvin Consulting Group activity. (30 min) 3. Review the metric system. Solve and check 1-4 Practice Problems. 4. Begin Introductory Metric System Lab. 5. Discuss scientific notation. (POSSIBLE) The Practice: In your practice packet: Any metric system practice & review examples The Assessment: METRIC SYSTEM QUIZ TOMORROW! Element Quiz on Friday! Sample Element Quiz Questions 1. 2. 3. 4. 5. 6. Co _______________________ Cr ________________________ Sn _______________________ Copper ____________________ Sodium ____________________ Iron ________________________ Moving the Decimal T // G // M // k h D base d c m // µ // n // p // f 1-4 PP#1 Convert 83 cm into meters. T // G // M // k h D base d c m // µ // n // p // f 0.83 meters 1-4 PP #2 Convert 459 L into milliliters. T // G // M // k h D base d c m // µ // n // p // f 459,000 mL 1-4 PP #3 Express 1123 pg in nanograms. T // G // M // k h D base d c m // µ // n // p // f 1.123 ng 1-4 PP #4 Express 0.032 m3 in liters. TRICKY! 1 cm3 = 1 mL Steps: 1. Convert 0.032 m3 to cm3. This equals mL. 2. Convert mL to L. TRY IT. HOW TO CONVERT 0.032m3 to L 1. Convert 0.032 m3 to cm3. a) Cubed conversions are different from simple meter to centimeter conversions. b) Move the decimal 2 spaces to the right to go from meter to centimeter, correct? NOT SO FAST! c) Move the decimal 2 spaces for EACH dimension. Since the unit is cubed, we’ll move the decimal 2 spaces to the right X 3! d) 0.032 m3 = 32,000 cm3 2. Convert mL to L. a) Since cm3 = mL, 32,000cm3 = 32,000 mL. b) Move the decimal 3 spaces to the left to go from milli to liters. c) 32,000 mL = 32 L 1-4 PP #5 Express 2.5 mm in micrometers. T // G // M // k h D base d c m // µ // n // p // f 2,500 micrometers 1-4 PP #6 Which is the longer amount of time: 1351 ps or 1.2 ns? A.) 1351 ps B.) 1.2 ns T // G // M // k h D base d c m // µ // n // p // f 1351 ps 1-4 PP #7 Which is the larger pressure: 232.1 kPa or 125,487 Pa? A.) 232.1 kPa B.) 125,487 Pa T // G // M // k h D base d c m // µ // n // p // f 232.1 kPa 1-4 PP #8 Which is the smaller mass: 285.0 cg or 23.78 dg? A.) 285.0 cg B.) 23.78 dg T // G // M // k h D base d c m // µ // n // p // f 23.78 dg 1-4 PP #9 Which is shorter: 175.6 mm or 38.4 cm? A.) 175.6 mm B.) 38.4 cm T // G // M // k h D base d c m // µ // n // p // f 175.6 mm 1-4 PP #10a 0.7824 mg to grams T // G // M // k h D base d c m // µ // n // p // f 0.000 782 4 grams 1-4 PP #10b 345,000 ng to grams T // G // M // k h D base d c m // µ // n // p // f 0.000 345 000 grams 1-4 PP #10c 0.00378 kg to grams T // G // M // k h D base d c m // µ // n // p // f 3.78 grams 1-4 PP #10d 34,981 micrograms to grams T // G // M // k h D base d c m // µ // n // p // f 0.034 981 grams •Lazy way to report really BIG or small numbers •Uses powers of ten rather than long strings of zeros •+ powers mean BIG numbers •- powers mean small numbers Expand or contract. 1. 250 = ______________ 2. 13,210,000 = ________ 3. 0.00150 = ___________ 4. 14 = ________________ 5. 0.00005 = ____________ 6. 1.6x10-4 = ____________ 7. 2.15x105 = ____________ 8. 1.0x101 = _____________ 9. 4.3x10-2 = ____________ Check your answers. 1. 250 = 2.5 x 102 2. 13,210,000 = 1.321 x 107 3. 0.00150 = 1.5 x 10-3 4. 14 = 1.4 x 101 5. 0.00005 = 5 x 10-5 6. 1.6x10-4 = 0.00016 7. 2.15x105 = 215,000 8. 1.0x101 = 10 9. 4.3x10-2 = 0.043 •Use the EE or EXP button to enter scientific notation. •NEVER use the ^ or x10. •Example: •Enter 6.02 x 1023 into your calculator. •Punch 6.02 as normal. •Then push the EE or EXP button. It replaces the x10. •Lastly, enter 23. •Summary: 6.02 EXP 23 1. 2. 3. 4. 6.02x1023 x 18.998 = ____________ 5.6x10-8 / 3.2x10-3 = _____________ 2.5x101 + 3.5x102 = _____________ 8.45x10-3 x 2.1x101 = ____________ 1. 1.144x1025 2. 1.75x10-5 3. 375 4. 0.17745 • Density is used to identify substances found in nature. • Density = mass/volume • Common units: g/mL or g/cm3 – – mL measures the volume of a liquid. It is NOT a cubed unit. cm3 measures the volume of a solid where length, width, and height were multiplied together. Example of Density: A rectangular sample is found. What is the density? 1. Measure the mass with a balance. 2. Measure the volume with a ruler since it has a normal (regular) shape. 3. Calculate. Another Example of Density: A strangely shaped sample is found. What is the density? 1. Measure the mass with a balance. 2. Measure the volume with a graduated cylinder using the water displacement method. 3. Calculate. A truth about the density of water: • A 1 cm3 box will hold EXACTLY 1 mL of water, and the 1 mL of water will weigh EXACTLY 1 gram! • Therefore, 1 cm3 = 1 mL = 1 gram. • You are going to have a chance to prove this in your lab today using a small blue solid (not hollow) cube. Density of a Metal Cube Lab Goals: Test the 1 mL = 1 cm3 rule and determine the type of metal that makes up your group’s cube using density. Steps: Follow the lab steps carefully, and be sure to record any measurements on your paper. You’ll have a very small lab report due on Monday. The specifics of the report are described on your lab handout. If you get stuck, send a group rep to Mrs. Tarvin. NOTE: The copy of the lab at your station should not leave the station. A copy of the lab is on the blog for your use at home. A student determines that a piece of an unknown material has a mass of 5.854 g and a volume of 7.57 cm3. What is the density of the material? (Density Practice Problems #1) A.) 0.773 g/cm3 B.) 1.29 g/cm3 C.) 44.4 g/cm3 D.) none of these A student determines that a piece of an unknown material has a mass of 5.854 g and a volume of 7.57 cm3. What is the density of the material? (Density Practice Problems #1) Steps: 1. Density = mass / volume 2. Mass = 5.854 grams; Volume = 7.57 cm3 3. Notice that the units will be grams/cm3. The problem doesn’t specify certain units, so I can use these. 4. 5.854 gram/7.57 cm3 = 0.773 g/cm3 Iron has a known density of 7.87 g/cm3. What would be the mass of a 2.5 dm3 piece of iron? Density Practice Problem #2 A.) 1.9675 grams B.) 19.675 grams C.) 196.75 grams D.) 19, 675 grams Iron has a known density of 7.87 g/cm3. What would be the mass of a 2.5 dm3 piece of iron? Density Practice Problem #2 Steps: 1. Density = mass / volume 2. Density = 7.87 g/cm3; Since the units are given for density, I am stuck with them. I cannot plug in a mass unless it is in grams. I cannot plug in a volume unless it is in cm3. 3. Convert 2.5 dm3 to cm3. Move the decimal to the right THREE spaces. 4. 7.87g/cm3 = mass / 2500 cm3 ; mass = 19,675 grams Mercury has a density of 13.5 g/cm3. How much space (in mm3) would 50.0 g of mercury occupy? Density Practice Problem #3 A.) 3.70 mm3 B.) 37.0 mm3 C.) 370.0 mm3 D.) 3,700 mm3 Mercury has a density of 13.5 g/cm3. How much space (in mm3) would 50.0 g of mercury occupy? Density Practice Problem #3 Steps: 1. Density = mass / volume 2. Density = 13.5 g/cm3; The mass MUST be in grams, and the volume MUST be in cm3. 3. 13.5 g/cm3 = 50.0 g / volume; REMEMBER – The volume will be in cm3 because of the density units. 4. ALGEBRA HELPFUL HINT: Put a 1 under the density & cross multiply. 5. 13.5 g/cm3 = 50.0 grams 1 volume (13.5 g/cm3)(volume) = (50.0 grams)(1) (13.5 g/cm3) (13.5 g/cm3) volume = 3.70 cm3 = 3,700 mm3 A sample has a mass of 1.02g and 3 a volume of 1.35cm , what is the density of the nickel? Density Practice Problems #4 A.) 0.756 g/cm3 B.) 1.38 g/cm3 C.) 1.32 g/cm3 D.) 7.56 g/cm3 What is the density of a material if its mass 2.02g and its volume is 0.500cm3? Density Practice Problem #5 A.) 1.01 g/cm3 B.) 4.04 g/cm3 C.) 0.248 g/cm3 D.) 4.48 g/cm3 Pure gold has a density of 19.32 g/cm3. How large (in dm3) would a piece of gold be if it had a mass of 318.97 g? Density Practice Problems #6 A.) 16.51 dm3 B.) 1.651 dm3 C.) 0.1651 dm3 D.) 0.01651 dm3 How many cm3 would a 55.932 g sample of copper occupy if it has a density of 8.92 g/cm3? Density Practice Problems #7 A.) 0.159 cm3 B.) 499 cm3 C.) 6.27 cm3 D.) 48.9 cm3 hey mrs tarvin, im sitting in my awesome chem lecture hall and we're doing review. i was wondering if you could remind me of what your sigfig tricks were to remember when to count them and when not to. thanks! alexandra ps, i hope you've got some great classes! I got this email yesterday during 4th pd from UGA Digits in measurement communicate valuable quantitative information. If you know your stuff, the digits can tell you qualitative information, too. Example: Compare the information in these two numbers. Don’t forget to read between the lines! a. 148,300 meters b. 148,336.420 meters Making Measurements 1. Examine the markings on the instrument. Note the smallest mark shown and the unit that you’ll be using. What decimal place does the smallest mark represent? 2. Measure the object as usual, and record all of the obvious markings. 3. THEN, ADD ONE ADDITIONAL DECIMAL PLACE TO YOUR MEASUREMENT. (ONE PAST THE MARKINGS OF THE INSTRUMENT.) Making Measurements 1. The ruler is marked to the nearest 1/10 of a centimeter. In other words, the smallest marking is 0.1 cm. 2. The object measures EXACTLY 5.7 cm according to the obvious markings. 3. In science, we record the one estimated digit beyond the obvious markings. We should record the measurement as 5.70 cm. (Note: Answer has ONE extra decimal place beyond the smallest marking.) Making Measurements What if you disagree with my estimate? What if you believe that the object is not EXACTLY on the 5.7 mark? Then, you would use a different estimated digit. Examples: 5.72 cm or 5.75 cm or 5.79 cm Analyzing measurement data: •Describing the instrument •Evaluating the “worth” Example: Consider the data recorded below. Length: 3.50 cm Width: 2.150 cm Could these have been made by the same instrument? How sensitive is the instrument? Some instruments are better than others, and we may all estimate different final digits in our measurements. Error is an important consideration in our measurements and calculations then. CONSIDER: What if you were asked to calculate the volume of a block? Volume includes THREE measurements (L x W x H). You could have THREE small errors factoring into your volume answer. Special rules exist in scientific calculations to prevent error from “snowballing” in our answers. Remember, measurements involve estimations, and that can be dangerous when working with volatile chemicals. Reducing the estimation risk: •When adding or subtracting: •Line up the decimals as usual. •Draw a vertical line at the end of the shortest #. •Add or subtract. Round the answer at the line. Addition and Subtraction Example 35.6 + 4.1 + 4.79 + 2 = Step 1: Line up the decimals. 35.6 4.1 4.79 +2 Step 2: Draw a vertical line at the end of the shortest #. 35.6 4.1 4.79 +2 Step 3: Add & round at the line. 46.49 = 46 1. 61.2 meters + 9.35 meters + 8.6 meters 2. 9.44 meters - 2.11 meters 3. 34.61 meters -17.3 meters 4. 8.3 meters x 2.22 meters 5. 8432 meters /12.5 1. 79.2 meters 2. 7.33 meters 4. 18 meters 5. 675 meters 3. 17.3 meters Reducing the estimation risk: •When multiplying or dividing: •Count the significant digits in all #’s. •Multiply or divide. Round the answer to the smallest number of sig figs. Learning to Count Sig Figs: Imagine the number inside the US. • Imagine the number inside the US. • If the decimal is PRESENT, go to the Pacific coast of the #. Look back across the # and begin counting digits at the 1st nonzero #. Don’t stop counting until you run out of digits no matter what! EXAMPLE: How many sig figs are in 0.0780400? P 0.0780400 123456 6 sig figs! A • Imagine the number inside the US. • If the decimal is ABSENT, go to the Atlantic coast of the #. Look back across the # and begin counting digits at the 1st nonzero #. Don’t stop counting until you run out of digits no matter what! EXAMPLE: How many sig figs are in 56,043,000? P 56,043,000 54321 5 sig figs! A Counting sig figs: 1. 0.05730 meter 2. 8765 meters 3. 0.00073 meters 4. 40.007 meters 5. 143 grams 6. 8.750x10-2 grams 7. 1.40x10-5 grams 1. 8.3 meters x 2.22 meters 2. 8432 meters /12.5 1. 18 meters 2. 675 meters Density Practice Problems 1. 2. 3. 4. 5. 6. 7. 0.773 g/cm3 19,675 g 3,700 mm3 0.756 g/cm3 4.04 g/cm3 0.01651 dm3 6.27 cm3 I know that you want me to solve them all with you so that you can correct your mistakes, BUT you need to give them ONE more shot first. Corrections due on Monday. A sample has a mass of 1.02g and 3 a volume of 1.35cm , what is the density of the nickel? Density Practice Problems #4 A.) 0.756 g/cm3 B.) 1.38 g/cm3 C.) 1.32 g/cm3 D.) 7.56 g/cm3 A sample has a mass of 1.02g 3 and a volume of 1.35cm , what is the density of the nickel? Density Practice Problems #4 What is the density of a material if its mass 2.02g and its volume is 0.500cm3? Density Practice Problem #5 A.) 1.01 g/cm3 B.) 4.04 g/cm3 C.) 0.248 g/cm3 D.) 4.48 g/cm3 What is the density of a material if its mass 2.02g and its volume is 0.500cm3? Density Practice Problem #5 Pure gold has a density of 19.32 g/cm3. How large (in dm3) would a piece of gold be if it had a mass of 318.97 g? Density Practice Problems #6 A.) 16.51 dm3 B.) 1.651 dm3 C.) 0.1651 dm3 D.) 0.01651 dm3 Pure gold has a density of 19.32 g/cm3. How large (in dm3) would a piece of gold be if it had a mass of 318.97 g? Density P P #6 How many cm3 would a 55.932 g sample of copper occupy if it has a density of 8.92 g/cm3? Density Practice Problems #7 A.) 0.159 cm3 B.) 499 cm3 C.) 6.27 cm3 D.) 48.9 cm3 3 cm How many would a 55.932 g sample of copper occupy if it has a density of 8.92 g/cm3? Density PP #7 Quick Check of Skills Solve the following on a ½ sheet of paper to turn in to Mrs. Tarvin in 15 minutes. 1. A) What is the volume (in cm3) of a block of gold whose density is 19.32 g/cm3 and mass is 48.6 grams? B) What is the volume in mm3? 2. What is the sum of 3.456m + 0.42m + 3.1m? 3. Divide: 79.23g / 6mL = 4. What is the most difficult thing that we’ve done so far? 1. A) What is the volume (in cm3) of a block of gold whose density is 19.32 g/cm3 and mass is 48.6 grams? 1. SET UP THE EQUATION. 2. CROSS MULTIPLY TO FIND THE DENOMINATOR. 3. USE THREE SIG FIGS IN YOUR ANSWER. 1. A) What is the volume (in cm3) of a block of gold whose density is 19.32 g/cm3 and mass is 48.6 grams? B) What is the volume in mm3? 1. USE THE ANSWER FROM A IN CM3. 2. MOVE THE DECIMAL ONE PLACE TO THE RIGHT TO GO FROM CENTI TO MILLI FOR EACH POWER. 3. THEREFORE, MOVE A TOTAL OF THREE SPACES TO THE RIGHT. 2. What is the sum of 3.456m + 0.42m + 3.1m? 1. LINE UP THE DECIMALS. 2. FIND THE SHORTEST NUMBER. (3.1) 3. DRAW A LINE AT THE END OF 3.1, WHICH MEANS THAT OUR ANSWER CANNOT GO BEYOND THE TENTHS PLACE. 4. ADD AND ROUND AT THE LINE TO THE TENTHS PLACE. 3. Divide: 79.23g / 6mL = 1. COUNT THE NUMBER OF SIG FIGS IN EACH NUMBER. 2. 79.23 HAS FOUR SIG FIGS. 3. 6 HAS ONLY ONE SIG FIG. 4. THEREFORE, THE ANSWER CAN HAVE ONLY ONE SIG FIG. 5. DIVIDE AND ROUND TO ONE SIG FIG. USE PLACE HOLDERS WHERE NEEDED IN FRONT OF THE DECIMAL. Tiered Measurement Lab • You’ll need paper, pencil, and a calculator. • You must apply metric conversions, measurement skills (using an estimated digit), density, and sig fig calculations. • Six stations are available, and each is worth a certain number of points. • You need to earn 60 points. No extra credit is available. • Record EACH measurement, and show ALL calculations. • Lab is due tomorrow. Use the data table to give you instructions. Be sure to label your work with station numbers. Record ALL measurements in each unit required. Show ALL calculations with each unit required. Convert the density of 0.58 g/mL to lb/gallon. (1 L = 1.057 qt and 4 qt = 1 gal; 1kg = 2.2 lb) Convert the density of 0.58 g/mL to lb/gallon. (1 L = 1.057 qt and 4 qt = 1 gal; 1kg = 2.2 lb) Convert the density of 0.58 g/mL to lb/gallon. (1 L = 1.057 qt and 4 qt = 1 gal; 1kg = 2.2 lb) Convert the density of 0.58 g/mL to lb/gallon. (1 L = 1.057 qt and 4 qt = 1 gal; 1kg = 2.2 lb) Convert the density of 0.58 g/mL to lb/gallon. (1 L = 1.057 qt and 4 qt = 1 gal; 1kg = 2.2 lb) If a pound of apples costs $0.79, then 5.3 lbs will cost _________. Give your answer to TWO decimal places which is customary with money. 1849 yards = __________ miles Hint: 5280 ft = 1 mile Give your answer to 4 sig figs. If Boston and New York City are 190 miles apart, then the distance between the two cities is _______ km. Hint: 1 km = 0.621 miles Give your answer to TWO sig figs. If a pound of apples costs $0.79, then a shopper with $2.00 will be able to purchase ________ lbs of apples. Give your answer to THREE sig figs. If a US car advertisement brags that an SVU gets 26 miles/gallon on the highway, then the same car would be described in Europe as getting ___________ km/L. Hint: 1 L = 1.057 qt; 4 qt = 1 gal; 1 km = 0.621 miles Give you answer to TWO sig figs.