10.1-10.3 Practice Test
advertisement

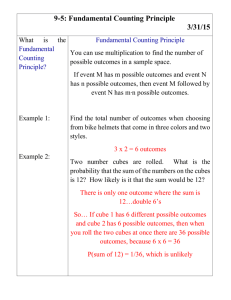
Name: ___________________________________ Block: _______________ Date: ___________________ Unit 10 Practice Test Objective 10.1: Interpret heating and cooling curves and phase diagrams. Directions: Use the cooling curve below to answer the following questions: 100 A 80 B C 60 Temperature (oC) 40 20 0 -20 -40 -60 -80 D E -100 F -120 0 2 4 6 8 10 12 14 16 Time (min.) 1. State the boiling point of the substance in Celsius: _________ 2. _________ Which segment represents the liquid being cooled? 3. Which segment represents freezing? _________ 4. How many minutes does it take from the time the substance begins to condense until it is completely liquid? ____ 5. Use your Chemistry Reference Tables to identify this substance: ____________________ Directions: Base your answers to the following questions on the phase diagram of substance Y below. 6. In what state is compound Y at 300 K and 150 atm? _________________ 7. What is the critical point of compound Y? ___________ __________ 8. What phase change does Y undergo if a sample at 100 K and 100 atm is heated to 250 K? ________________________________ 9. The phase change in #7 is (exothermic/endothermic). 10. What is the boiling point of compound Y at 150 atm? _______________ Score box A= 10/10 (100) B= 9/10 (90) NY Objective 10.2: Calculate the heat lost or gained in temperature changes and phase changes. Directions: Solve the following problems, including the correct FORMULA, WORK, UNITS, and SIG FIGS. Place a BOX around your final answer. 1. During a chemical reaction, the temperature of 9.07 g of liquid water goes from 21.2°C to 16.5oC. Calculate q. [2] 2. How much heat is needed to melt 0.56 grams of ice at 0oC? [2] 3. What is the specific heat of a metal if 205.4 grams of it increases in temperature from 10.0oC to 30.0oC from the addition of 575 J of heat energy.[2] 4. Identify the metal in question #3: ________________________________[1] 5. While planting a tree in his backyard, Pete uncovers a large nugget of gold! If it takes 1.5 kJ of heat to change its temperature from 11°C to 96ºC, what is the mass of his gold nugget?[2] The following questions involve the use of your Reference Tables. 6. A student adds 300 Joules of heat to two metal cubes of the same mass, which are both at 25oC. One cube is made of nickel, while the other is made of zinc. Both cubes receive the exact same amount of heat. Which of the following is true after the cubes absorb heat? A. B. C. D. The nickel cube will have a higher final temperature than the zinc cube. The zinc cube will have a higher final temperature than the nickel cube. Both cubes will have the same final temperature. There is not enough information to tell. 7. Discuss your rationale for your answer to question #6. Score box A= 10/10 (100) B= 9/10 (90) NY Objective 3: Interpret a potential energy diagram. (p. 498-500) ( kJ ) Directions: The diagram below is a potential energy diagram for the overall reaction: H2 + Cl2 2HCl 2HCl H2+ Cl2 1. Give the following values in kilojoules (kJ): a) Activation energy, Ea (forward reaction) _________________ b) Heat of reaction (∆H) _________________ c) _________________ Potential energy of the products d) Potential energy of the activated complex _________________ 2. Determine whether the following statements about the reaction 2HCl H2 + Cl2 are TRUE (T) or FALSE (F) based on the PE diagram above. a) b) c) d) The reaction is endothermic. The product molecules are less stable than the reactant molecules. PEproducts > PEreactants The bonds in the reactant molecules are stronger than the bonds in the product molecules. 3. A student adds 5.0 grams of a salt to a 100 mL of water at 22oC and monitors the temperature of the water as he dissolves it in the water. His data is shown below: Time (s) Temperature (oC) 0 30 60 90 120 22.0 28.2 30.3 31.1 31.2 Discuss whether the dissolving of this salt is an endothermic or exothermic process. [2] Score box A= 10/10 (100) B=9/10 (90) NY