Preview - West Ada School District
advertisement

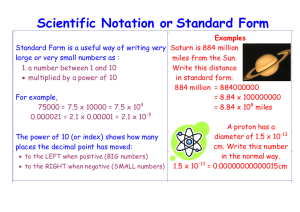
ANNOUNCEMENTS: • RED RIBBON WEEK! TOMORROW: SUPERHERO DAY! • Substitute Teacher & Assembly Friday, November 6th, 2015 (I will be in building) • NO SCHOOL (Veteran’s Day) __________________________________________________________________________________________________ Why does Matter, matter? Sub-question: What is the importance of units in science? Essential Question: PREVIEW: Read the following passage and work the problems below… THE IMPORTANCE OF UNITS: When crossing the border into Canada, American motorists are often surprised to see speed limits of “90” or “100”. If they don’t realize that the Canadians measure speed in kilometers/hr while Americans measure in miles/hr (1.00 mile/hr = 1.61 kilometers/hr; 60 miles/hr = 97 km/hr) they may soon be in trouble with the law. If, for example, an American motorist accelerated until her speedometer (measured in miles/hr) reaches “100,” she will be traveling 38 miles/hr over the posted speed limit of 100 km/hr since the speed of 100 km/hr is equal to only 62 miles/hr. As this example illustrates, measurements without units are meaningless and may lead to serious misunderstandings. Everything that can be measured must be expressed with appropriate units. UNITS IN EVERYDAY LIFE: We use units every day, often without even realizing it. In the statements that follow, you will find a wide variety of interesting facts, but each is missing a crucial piece of information – the dimensions (units)! All the statements are meaningless until you supply the appropriate units. On the basis of your experiences, try to match the appropriate units from the list provided. ID CCSS STANDARDS: 6.S.2.1.3 Compare densities of equal volumes of a solid, a liquid, or a gas. 6.S.2.1.4 Describe the effect of temperature on density. In journal: Complete the following by matching the appropriate units from the list provided. carats inches megabars tons grams/mL liters stories feet kilowatt-hours pounds degrees Fahrenheit kilometers milligrams degrees Celsius kilograms miles per hour cm kcal (Cal) miles yards 1. America’s tallest building (One World Trade Center in New York) is 104 ______ high. 2. The Amazon River in South America is 6296 ____ long. 3. The highest recorded temperature in the United States was in Death Valley, California, when the mercury reached 57 ____! 4. The world record rainfall occurred in Cherrapunji, India, where 1042 ____ of rain fell in one year. 5. The largest recorded hailstone to ever fall landed in Coffeyville, Kansas, in 1979. It had a diameter of 44.5 ____! 6. The Empire State Building in New York is 1250 _____ high. 7. The Nile is the world’s longest river. It is 4180 ____ long. 8. The longest punt in NFL history was by Steve O’Neal of the New York Jets. He kicked the football 98____. 9. The largest seed in the world is that of the coc-de-mer coconut tree, which may weigh as much as 40 ___! 10. The world’s largest meteorite is located in Southwest Africa. It weighs 650 ____. 11.The most popular soft drink in the world is currently Coca Cola®. More than 210 million ____ were consumed each day in 1990. 12.The largest diamond in the world was mined in from South Africa in 1905 and weighs 1306 _____. 13.Earth is the densest of the nine planets, with an average density of 5.515 ______. 14.The world’s fastest aircraft is the Lockheed SR-71 Blackbird, clocking a record speed of 2,193.67 _____. 15.The largest gold nugget ever found had a mass of 100 _____! 16. One large chicken egg contains an average of 274 ____ cholesterol. 17. A 16-year old male requires an average of 2800 ______ of energy per day while an average 16-year old female requires 2100 ____. 18. The United States produces & consumes more electric energy than any other nation. Each year the United States produces over 2500 billion ____. 19.The largest pressure ever developed in a laboratory was 1.70 _____, used to solidify hydrogen in 1978. 20. The coldest temperature ever recorded was -128.6 ____ in Vostok, Antarctica, in 1983. ID CCSS STANDARDS: 6.S.2.1.3 Compare densities of equal volumes of a solid, a liquid, or a gas. 6.S.2.1.4 Describe the effect of temperature on density. PREVIEW: What does SI Units stand for? International System of Units: a system of physical units (SI Units ) based on the meter, kilogram, second, ampere, kelvin, candela, and mole, together with a set of prefixes to indicate multiplication or division by a power of ten. Factor Table 1: SI Prefixes and Symbols Decimal Representation Prefix Symbol 1018 1,000,000,000,000,000,000 exa E 1015 1,000,000,000,000,000 peta P 1012 1,000,000,000,000 tera T 109 1,000,000,000 giga G 106 1,000,000 mega M 103 1,000 kilo k 102 100 hecto h 101 10 deka da 100 1 10-1 0.1 deci d 10-2 0.01 centi c 10-3 0.001 milli m 10-6 0.000 001 micro m 10-9 0.000 000 001 nano n 10-12 0.000 000 000 001 pico p 10-15 0.000 000 000 000 001 femto f 10-18 0.000 000 000 000 000 001 atto a https://www.youtube.com/watch?v=7N0lRJLwpPI https://www.youtube.com/watch?v=7N0lRJLwpPI ID CCSS STANDARDS: 6.S.2.1.3 Compare densities of equal volumes of a solid, a liquid, or a gas. 6.S.2.1.4 Describe the effect of temperature on density. REVIEW/PREVIEW: What is a law? Law: A rule describing a consistent pattern in Nature. It happens every time all the time. What does conservation mean? Conservation is the act of preserving, guarding, or protecting. What is mass? Mass: the amount of matter a substance has. What is the Law of Conservation of Mass? Law of Conservation of Mass - a fundamental principle of science that states that matter cannot be created or destroyed in an isolated chemical reaction -- only changed. ID CCSS STANDARDS: 6.S.2.1.3 Compare densities of equal volumes of a solid, a liquid, or a gas. 6.S.2.1.4 Describe the effect of temperature on density. PREVIEW: What is the difference between an element and a compound? Chemical Element -- a pure chemical substance consisting of a single type of atom, distinguished by its atomic number, found on the Periodic Table of Elements. Chemical Compound -- a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Draw a Venn Diagram Comparing/Contratins the two: Element: Compound: ID CCSS STANDARDS: 6.S.2.1.3 Compare densities of equal volumes of a solid, a liquid, or a gas. 6.S.2.1.4 Describe the effect of temperature on density. PREVIEW: What is the smallest unit of each an element and a compound called? The smallest unit of elements are called atoms. The smallest unit of compound are called molecules. PREVIEW: Exothermic Reactions: reactions produce heat. Endothermic Reactions: require heat to enable the reaction to occur. What is a catalyst? Catalyst – substance/energy source that increases the rate of or triggers a chemical reaction without itself undergoing any permanent chemical change. What does it mean by a yield during chemical reactions? In chemistry, yield, also referred to as reaction yield, is the amount of product obtained in a chemical reaction of the reactants. (like “equals” in math) Photosynthesis Reaction: ID CCSS STANDARDS: 6.S.2.1.3 Compare densities of equal volumes of a solid, a liquid, or a gas. 6.S.2.1.4 Describe the effect of temperature on density. SUNLIGHT (catalyst) __ H2O + __ CO2 WATER __ C6H12O6 + __ O2 GLUCOSE CARBON DIOXIDE REACTANTS “YIELDS” PRODUCTS List as many types of chemical change as you can. ID CCSS STANDARDS: 6.S.2.1.3 Compare densities of equal volumes of a solid, a liquid, or a gas. 6.S.2.1.4 Describe the effect of temperature on density. OXYGEN