In situ training of free-ranging predatory lizards reduces their
advertisement
Ecological immunisation: In situ training of free-ranging predatory lizards
reduces their vulnerability to invasive toxic prey
Electronic Supplementary material (ESM)
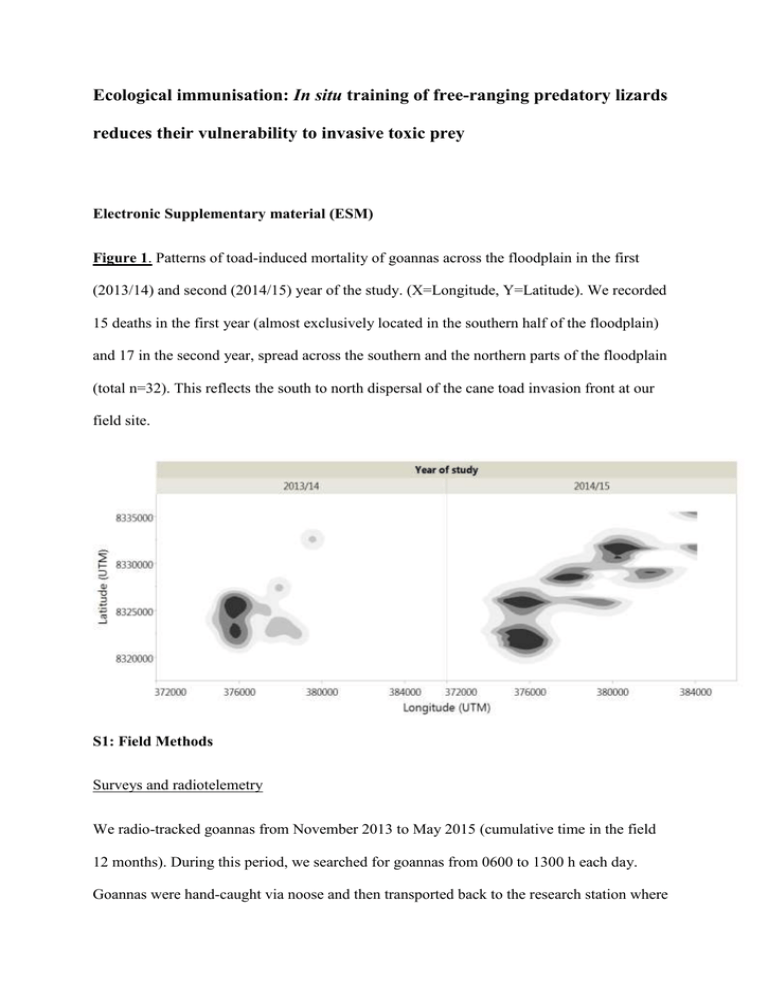
Figure 1. Patterns of toad-induced mortality of goannas across the floodplain in the first
(2013/14) and second (2014/15) year of the study. (X=Longitude, Y=Latitude). We recorded
15 deaths in the first year (almost exclusively located in the southern half of the floodplain)
and 17 in the second year, spread across the southern and the northern parts of the floodplain
(total n=32). This reflects the south to north dispersal of the cane toad invasion front at our
field site.
S1: Field Methods
Surveys and radiotelemetry
We radio-tracked goannas from November 2013 to May 2015 (cumulative time in the field
12 months). During this period, we searched for goannas from 0600 to 1300 h each day.
Goannas were hand-caught via noose and then transported back to the research station where
we measured their mass and snout to vent length (SVL). We then attached radio transmitters
(Holohil, RI-2B VHF radio transmitters) to their tail following methods in previous studies
(1), and returned them to their initial point of capture for release within 24 h. Mortality
sensors in the radio transmitters triggered after 12 h of non-activity. We attached a thermal
data-logger (Thermochron TC, OnSolution Pty. Ltd.) to each transmitter, to measure ambient
temperatures; these data also pin-pointed time and date of death for animals that died between
field-trips. Marked goannas were radio-located at least once every two to three days on each
trip.
Conditioned Taste Aversion training
We exposed free-ranging goannas to small live toads (30 – 70 mm snout-urostyle length
[SUL], 10 to 25 g), <2% of the lizard’s body mass (criterion for non-lethality, based on
laboratory trials; (2)). To reduce toxin content, we squeezed the toads’ parotoid glands prior
to trials.
The “teacher toad” was presented on fishing line attached to a 4-m fishing rod, with the toad
attached to the fishing line via a cotton-thread waist belt (such that the toad was easily
detached if seized by the lizard). We approached foraging goannas during the day and
dropped the toad to the ground within a metre of the goanna’s head. If the toad was ignored,
the procedure was repeated ten minutes later (up to three times each trial). We attempted to
watch interactions with the toad, but from far enough away that the lizard was undisturbed.
We scored whether each goanna ignored the toad, or responded by approaching it, tongueflicking, biting or eating. Training trials were concurrent with radiotelemetry, conducted
opportunistically with every goanna.
This study abided by strict ethical guidelines and we adopted several methods to minimise the
suffering of the animals involved. Unless a toad was attacked by a goanna, it did not sustain
any injuries during trials. Attacks were rapid and almost instantly fatal to the toad. Most
attacks ended in consumption; the few toads that were only bitten were humanely euthanized
immediately thereafter. Each toad was used in only a single trial; if not consumed, it was
euthanized humanely. Prior to trials, toads were housed in high quality conditions with
excellent husbandry.
S2: Overall survival estimates in program MARK
Based on their presence or absence on each field trip, we scored encounter histories for each
individual goanna across eleven occasions (field trips) and corrected for time intervals
between trips. We initially assessed the goodness-of-fit of a fully time-dependent Cormack–
Jolly–Seber (CJS) model, using Test2 and Test3 of program RELEASE implemented in
MARK (3). These tests did not indicate any significant lack of fit of the CJS model (P >
0.29), suggesting that the CJS model offers an appropriate framework to estimate survival
and recapture rates. Therefore, we fitted eight CJS models in which survival (phi) and
recapture rates (p) were either held constant, varied over time, or varied as a function of
training, location at the field site (North or South) or an interaction between training and
location. As the best initial model contained p(t), we did not pursue any models that included
main affects and p(.). This analysis included records of all telemetered individuals regardless
of their fate (i.e. records were not censored).
We used Akaike Information Criterion to identify the best supported model in the MARK
analysis (4) (Table 1.). This model included the training status of the goanna (trained vs.
untrained), the location of the goanna (north vs. south i.e. as a proxy for toad exposure) and
the interaction between training and location. Untrained goannas in the south (i.e. the area
with longest exposure to toads) had the lowest rates of survival. Survival rates of trained
goannas in the south were similar to those of both untrained and trained goannas in the north
of the floodplain (Table 2 and Figure 2). Hence, we excluded the northern population from
subsequent analyses.
Table 1. Models run in MARK and their associated AICc and Delta AIC values. (T = training
status, L = location on floodplain i.e. North vs South, I = training * location interaction term)
Model
AICc
Delta AIC
{Phi( T + L + I ) p(t) PIM}
521.6507
0
{Phi( T + L ) p(t) PIM}
523.60004
1.9497
{Phi( L ) p(t) PIM}
523.3116
4.6609
{Phi(t) p(t) PIM}
532.8191
11.1684
{Phi( T ) p(t) PIM}
535.2775
13.6268
{Phi(t) p(.) PIM}
535.5416
13.8909
{Phi(.) p(t) PIM}
538.5761
16.9254
{Phi(.) p(.) PIM}
543.2208
21.5701
Table 2. Overall survival estimates for trained and untrained goannas in the North and South
of the floodplain.
Category
Annual survival
SE
Untrained South
0.0029
0.0028
Trained South
0.111
0.08
Untrained North
0.162
0.075
Trained North
0.192
0.18
Figure 2. Estimates of annual rates of survival of all goannas (trained and untrained) in
the North and South using MARK. This analysis included all individuals regardless of
their training status, tracking history or ultimate fate.
References
1.
Ujvari B, Madsen T. Increased mortality of naive Varanid lizards after the invasion of non-
native cane toads (Bufo marinus). Herpetological Conservation and Biology. 2009;4(2):248-51.
2.
Pearson DJ, Webb JK, Greenlees MJ, Phillips BL, Bedford GS, Brown GP, et al. Behavioural
responses of reptile predators to invasive cane toads in tropical Australia. Austral Ecology.
2014;39(4):448-54.
3.
Amstrup SC, McDonald TL, Manly BFJ. Handbook of Capture-Recapture Analysis: Princeton
University Press; 2010.
4.
Burnham KP, Anderson DR. Multimodel inference - understanding AIC and BIC in model
selection. Sociological Methods & Research. 2004;33(2):261-304.




