MI11_Presentation
advertisement

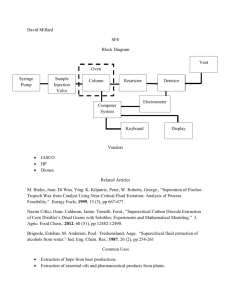
𝟐 ′′ Vibronic Analysis of the 𝑨 𝑬 State of NO3 Terrance J. Codd*, John Stanton†, and Terry A. Miller* * The Laser Spectroscopy Facility, Department of Chemistry and Biochemistry The Ohio State University, Columbus, Ohio † Department of Chemistry, The University of Texas at Austin, Austin, Texas Previous Work A E NO3 2 Hirota and colleagues reported observation of the 401 and 201 bands of the electronically forbidden A 2 E X 2 A2 transitiona First broad range spectrum was taken by Deev et al. in an ambient CRDS experimentb Several bands were assigned in this work and evidence of strong JT coupling was reported 2 2 Jacox and Thompson recorded FTIR spectra of the A E X A2 transition in a Ne matrix experimentc It significantly extended the spectral range and made several more assignments They reported evidence of weak JT coupling in 4 Most recently, Takematsu et al. have reported the observation of the vibronically forbidden origin of the A 2 E X 2 A2 transition and observed several hot bandsd They refined the position of the origin band to 7062.25 cm-1 and reported a second peak roughly 8 cm-1 to the blue a. K. Kawaguchi, T. Ishiwata, E. Hirota, I. Tanaka. Chem. Phys. 231, 193 (1998). E. Hirota, T. Ishiwata, K. Kawaguchi, M. Fujitake, N. Ohashi, I. Tanaka. J. Chem. Phys. 107, 2829 (1997) b. A. Deev, J. Sommar, M. Okumura. J. Chem. Phys, 122, 224305 (2005). c. M. E. Jacox, W. E. Thompson. J. Phys. Chem. A, 114, 4712-4718 (2010). d. K. Takematsu, N. C. Eddingsaas, D. J. Robichaud, M. Okumura, Chem. Phys. Lett., 555, 57-63 (2013) The “important” electronic states of NO3 THE A-X ELECTRONIC SPECTRUM OF NO3: SOME THEORETICAL RESULTS AND IDEAS John F. Stanton and Christopher S. Simmons 66th OSU International Symposium on Molecular Spectroscopy, TJ03, June 20-24 ,2011 X̃2A2′ 4e 1e 1a2 2 2 1 X̃2A2′ 0 cm1 4e 1e 1a2 2 1 2 7064 cm1 Ã2Eb′′ 4e 1e 1a2 1 Ã2Ea′′ 2 15105 cm1 B̃2Ea′ 2 B̃2Eb′ Ã2Ea′′ Ã2Eb′′ B̃2Ea′ B̃2Eb′ The “important” electronic states of NO3 THE A-X ELECTRONIC SPECTRUM OF NO3: SOME THEORETICAL RESULTS AND IDEAS John F. Stanton and Christopher S. Simmons 66th OSU International Symposium on Molecular Spectroscopy, TJ03, June 20-24 ,2011 X̃2A2′ 4e 1e 1a2 2 2 1 Ã2Ea′′ Ã2Eb′′ B̃2Ea′ 3 ,4 X̃2A2′ B̃2Eb′ 3 ,4 0 cm1 4e 1e 1a2 2 1 2 7064 cm1 Ã2Eb′′ 4e 1e 1a2 1 2 15105 cm1 2 JT Ã2Ea′′ JT 2 JT B̃2Ea′ 2 B̃2Eb′ JT A true multistate, multimode system with rich spectra and plenty of unsolved problems! NO3 Vibronic Structure and Transitions Vibronically allowed transitions: e" v " v e v A1 e (ground state) e" v " e or Symmetry of electric dipole: or 𝑒′′ 𝑎2′′ 𝑎1′′ 1 Mode 1 2 3 4 Symmetric stretch Umbrella oop bend Antisymmetric stretch Antisymmetric ip bend 3 Symmetry D3h a1 ' 42 a2 " 11 e' 𝑒′′ 21 e' 41 ~ 0 A 2 E" 0 ~ X 2A2' ≈≈ ≈ ≈ 𝜇𝑒(∥) 𝜇𝑒(⊥) 𝜇𝑒(∥) MR-JC-CRDS Experimental Setup SRS (1 m, 18 atm H2) 20 Hz, 8ns, 500 mJ Nd:YAG pulse laser 20 Hz, 8ns, 100 mJ Filters Sirah Dye Laser Raman Cell 1st or 2nd Stokes 2-10 mJ,Δν~3 GHz Ring-down cavity with slit-jet (absorption length ℓ = 5 cm) L = 67 cm PD InGaAs Detector 20 m Fiber Optic ℓ R ~ 99.995 – 99.999% @ 1.3 m Vacuum Pump Collimator Room Temperature vs Jet-Cooled Spectra Room Temperature Jet Cooled 7700 7500 7500 7800 7600 7600 7900 7700 7700 Room temperature data from: A. Deev, J. Sommar, M. Okumura. J. Chem. Phys, 122, 224305 (2005). 8000 7800 7800 8100 7900 8100 8200 8000 8200 wavenumber wavenumber wavenumber 8300 8100 8300 840 820 8400 a.u. Jet-Cooled CRDS Data 7600 7800 8000 8200 8400 8600 a.u. wavenumber (cm-1) 8800 9000 9200 wavenumber (cm-1) 9400 9600 Vibronic Hamiltonian, H ev , for Nuclear Motion on the Electronic Potential Energy Surface, V H ev TˆN V 3D Plot of V showing Jahn-Teller Distortion Quadratic Vibronic Hamiltonian H ev TˆN p 3 N 62 p p 1 1 2 i | Qi | i | Qi ,r |2 2 i 1 r , 2 i 1 kQ i 1 r , i i ,r Harmonic Oscillator Linear Jahn-Teller p 1 gii (Qi ,r ) 2 i 1 r , 2 p p , j i i 1 s j 1 1 cij (Qi ,r Q j ,r ) 2 r , p 1 bij Qi ,r Q j 2 i 1 r , j 1 T. A. Barckholtz, T. A. Miller, Int Rev in Phys. Chem.17, 435-524 (1998) Quadratic Jahn-Teller Cross-Quadratic Jahn-Teller Bi-linear Coupling Vibronic Parameters Hamiltonian Parameters 2V i Q Q i , i , 0 V bij Q Q i , j 2 V ki Q i , 0 0 2V gii Q Q i , j , 0 2V cij Q Q i , j , 0 Experimental Parameters ki2 M i Di 2 i3 Ki gii i 2 ′′ 𝐴 𝐸 State Vibronic Interactions | 0,1,0,0 | 0,0,1,1 K3,K4 b1,3 | 1,0,0,1 b1,4 K3,K4 b1,4 | 1,0,1,0 c3,4 D3 D4 c3,4 K3 D3 K4 b1,3 | 0,0,1,0 c3,4 | 0,0,0,1 K3,K4 D4 | 0,0,0,0 | | 1 , 2 , 3 , l3 , 4 , l4 Vibronic Assignments 4 02 a.u. 4 210 1 0 210 410 110 210 310 110 410 7600 7800 8000 8200 8400 8600 wavenumber (cm-1) 210 430 1 0 a.u. 24 4 3 0 310 410 8800 2 30 2 0 1 2 0 0 14 210310 410 2 02 410 210310 102 410 310 402 1 2 2 0 30 wavenumber (cm-1) 9000 9200 9400 4 50 2 30 410 9600 Complementary of Parallel and Perpendicular Bands This means that we can use (41 vibronic levels x a2) a1 the observed perpendicular combination bands to find the position of the 𝑒′′ components of the degenerate modes. e 2 4 1 1 a2 = 21 vibrational frequency (vibrational symmetry a2) e 21 a1 e 1 4 ~ A 2 E" a2 e e e ≈ ≈ ~2 ' X A2 ≈ Comparison of Observed and Simulated Line positions 4 02 210 210 410 a.u. 4 1 0 110 210 310 110 410 7600 7800 8000 8200 wavenumber 8400 8600 Comparison of Observed and Simulated Line positions 210 430 1 0 a.u. 24 4 8600 3 0 2 0 1 2 0 0 14 210310 2 02 410 310 410 8800 2 30 210310 410 1 2 0 0 23 4 50 2 30 410 310 402 102 410 9000 9200 wavenumber 9400 9600 Comparison of Observed and Calculated (John Stanton) Line Positions (Parallel Bands Only) Further work clearly needed Conclusions • Over 20 Vibronic Bands in the A 2 E X 2 A2 Electronic Transition have been Observed and Assigned • The Structure of the A 2 E State has been Well Stimulated Including Linear and Quadratic Vibronic Interaction Terms • Harmonic Frequencies for All 4 Vibrational Modes and JahnTeller Parameters for the e' Modes have been Obtained • More Detailed Comparison to Calculations Forthcoming Imminently