Preparing Solutions
advertisement

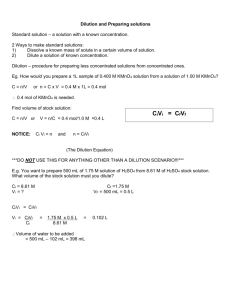
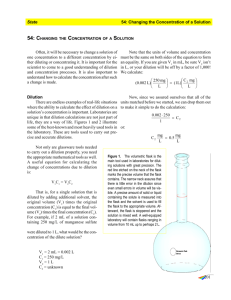
Preparing Solutions Standard Solutions Solutions can be prepared two ways: i) solids added to liquids OR ii) liquids added to liquids. Standard Solution: A solution for which the precise concentration is known. (Prepared in labs and industry.) We will be preparing a standard solution by accurately massing a solute then carefully and gradually adding a precise amount of water. Solution Preparation Stock Solution: a solution, usually concentrated or saturated, that is in stock or on the shelf and available for use. Dilution: the process of decreasing the concentration of a solution, usually by adding more solvent. We will be preparing a standard solution by accurately measuring a volume of stock or standard solution then carefully and gradually adding a precise amount of water. Required Equipment: • Volumetric flask – comes in different sizes and has one precise marking, unlike an Erlenmeyer flask that has approximate volume markings. • Precise electronic balance – to mass the solid that will be put into a volumetric flask to make a standard solution • Pipette – for adding a very accurate amount of stock solution to a volumetric flask and then diluting the initial solution to get a new standard solution. Pipettes Preparing a Standard Solution - Technique 1) Mass the solute on wax weighing paper, or plastic container & place the solute in a VERY CLEAN beaker 2) Rinse off the weighing paper or plastic container with distilled water to ensure all the massed solute molecules end up in the beaker 3) Add some distilled water & stir the solute MAKE SURE all the solute is DISSOLVED! 4) Once dissolved transfer the solution into a volumetric flask using a funnel and ENSURE NOT A DROP is spilled or lost 5) Rinse out the beaker 3 times with distilled water starting from the top and transfer the wash into volumetric flask Technique 6) Rinse out the funnel & rinse off the tip when removed 7) Add distilled water until 2-3 centimeters BELOW the mark, put a stopper on & invert the flask several times to mix it well 8) Looking at the line on the volumetric flask at eye level, add the distilled water DROP by DROP up to the line until the bottom of the meniscus is sitting on the line. You can use a dropper or small pipette to do this. 9) The solution must be stored with a stopper to avoid impurities. BEFORE using the solution, it will need to be shaken or inverted several times to ensure all the solute is dissolved since it has been sitting still for a while. http://www.youtube.com/watch?v=cckAwavEKA0 Calculations Starting with Mass What is the mass of NaOH pellets needed to make 750 mL of a 3.00 M solution? Given: V = 750 mL = 0.750 L M or c = 3.00 M = 3.00 mol/L 1st find n = ? n = c x V (or M x V) 2nd find m = ? Calculations Starting with Mass What is the mass of NaOH pellets needed to make 750 mL of a 3.00 M solution? Given: V = 750 mL = 0.750 L M or c = 3.00 M = 3.00 mol/L 1st find n = ? n = c x V (or M x V) 2nd find m = ? m = n x Molar mass n= c x V = # mol n = 3.00 mol/L x 0.750 L = 2.25 mol m = 2.25 mol x 40.0 g/mol = 90.0 g Dilution Calculations When determining the volume needed of a stock solution to make a new solution, just remember that original volume of solution has the same number of moles as the final solution, only added H2O… moles solute BEFORE = moles solute AFTER the dilution n before = n after the dilution Dilution Calculations moles solute BEFORE = moles solute AFTER the dilution n= CxV n=MxV n before = n after C1 x V1 = C2 x V2 Mi x Vi = Mf x Vf the dilution OR Dilution Calculations: Example Water is added to 0.200 L (Vi ) of a 2.40 mol/L (Mi) NH3(aq) cleaning solution, until the final volume is 1.000 L (Vf). Find the molar concentration of the final, diluted solution. Dilution Calculations: Example Water is added to 0.200 L (Vi ) of a 2.40 mol/L (Mi) NH3(aq) cleaning solution, until the final volume is 1.000 L (Vf). Find the molar concentration of the final, diluted solution. Only one equation needed n before = Ci x Vi Mi x Vi = = n after Cf x Vf Mf x Vf OR 2.40 mol/L x 0.200 L = Mf x 1.000 L Mf = 2.40 mol/L x 0.200 L 1.000 L Mf = 0.480 mol/L Dilution Calculations: Example Water is added to 0.200 L (Vi ) of a 2.40 mol/L (Mi) NH3(aq) cleaning solution, until the final volume is 1.000 L (Vf). Find the molar concentration of the final, diluted solution. NEED: Mf = ? Mi x Vi = Mf x Vf Mf = Mi x Vi Vf Mf = 2.40 mol/L x 0.200 L 1.000 L Mf = 0.480 mol/L Preparing a Standard Solution by Dilution 1) Using calculations, determine the amount of stock solution required to make the new solution and choose an appropriately-sized pipette. Example, the stock solution is 2.00 mol/L and the diluted solution needs to be 0.040 mol/L in a 1.00 L volumetric flask. Vi = Mf x Vf Mi = 0.040 M x 1.00 L 2.00 M = 0.020 L (20 mL) 2) Choose a 20 mL pipette or a graduated pipette that holds 20 mL of solution Preparing a Standard Solution by Dilution 3) Using the bulb, fill the pipette with solution a few centimeters above the marked amount. Quickly replace the bulb with your thumb 4) Carefully, slide or loosen your thumb until you see the level of the solution lowering at a slow rate. Stop at the marked line. You may have to try a few times since using a bulb and pipette effectively takes practice. 5) Once the bottom of the meniscus is at the line, press firmly with your thumb to hold that level and carry the pipette to the volumetric flask. Release the solution into the flask. 6) Fill the volumetric flask carefully as explained in technique section of preparing a Standard Solution from Mass http://www.youtube.com/watch?v=3EOiCrtvUUM HOMEWORK • • • • • Read page 300-307 pg 302 # 1,2,4,5a pg 306 # 6-8 pg 306-307 Questions # 2 – 5, 8 (skip d :) READ OVER Activity 6.5.1 pg 301 & Activity 6.5.2 pg 305