Lab Safety
advertisement

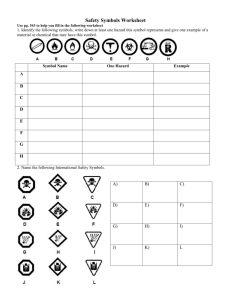
Coach Tate Biology/Pre-AP Biology Room D110 International Studies Academy Ninth Grade Center Week at a Glance Monday: Pre-Assessment and Supply List , Homework Assignment #1 “ About Me” * and Supplies (due 8-28-13), Tuesday: Work-styles Survey, Study-styles List Wednesday: Course Syllabus overview, Class Procedures/Daily Routine, Safety Controls Homework Assignment #2 “ Safety Symbols” , Signed Course Syllabus, Unit One Vocabulary* , safety contract Thursday: Science Journal Set Up; Lab Safety PowerPoint and Video Friday: Lab Equipment ; Scientific Method PowerPoint * = journal entry Monday 8-26-13 Objective: To asses students for prior knowledge of life science content. Clip: Intro to Biology Brief Instructor Introduction ( 3 min) About Me Homework Assignment( 3 min) Supply List ( 3 min) Pre-Assessment ( reminder of class) Intro to Biology http://www.youtube.com/watch?v=VgTPg9 9V_JM Meet Ms. Tate (About Me) Born and raised in St. Louis, Missouri, I attended Parkway South High where I participated in track and field all four years. I also was in a scholars club called MLABSE Scholars. Keeping good grades, I received a full academic scholarship to the University of Arkansas at Pine Bluff, where I majored in Biology/Pre-Med. After graduating from undergraduate school, I decided to go into education. I have been teaching science since 2005. This will be my 9 th year teaching high school science. I have a handsome 7 year old son (Alexander) and a 11 year old step daughter (BrTanyon). I love sports, cooking, forensics and reading books of different sorts. I also have pit bull named Grady. Course Supply List Materials needed for class: Pens (black or blue ink), Sharpened #2 Pencils A box of Kleenex Notebook paper; Two Pocket folder 2 composition notebooks Hand sanitizer Materials needed for home: Colored Pencils/Crayons Scissors Glue Sticks Construction Paper Biology Pre-Assessment Please do not write on the test. BRUNCH Method Breakdown illustrations/tables/figures Read, then read again ( the directions and question) Underline the sentence, highlight or bracket important terms Notes, make them on the side. Cancel out the two incorrect answers Hand pick the BEST answer choice. Tuesday 8/27/13 Students will take an assessment to understand their individual learning styles and complete a list of study styles based on what they have learned about how they learn best. Engage: What Type of Learner are You? (video clip)http://www.youtube.com/watch?v=zkE0sI7a_GA 1. Work Styles Survey ( 10) min- circle, code color then tally up) *****Create Your Learner Stick ***** 2. Work Styles PowerPoint ( separate from this one)- (15min) 3. Study Styles List ( as a class) http://www.youtube.com/watch?v=cgPfyPCXyU * Reminder Homework ( About Me Journal Entry and Supplies due tomorrow!) Wednesday 8-28-13 Students will review over the course syllabus for biology; Students will demonstrate an understanding of the class procedures/Daily Routine Agenda 1. Seating Chart 2. “About Me” Journal Entries 3. Review over course procedures and course syllabus ( student checklist) 4. Safety Contract 5. Questions??? Homework: Parent/Student contact sheet ( due Friday) Safety laboratory safety contract (only return the bottom portion; top portion attached to journal (3R)- (due Friday) Safety symbols( 4L)- (due Tuesday), Vocabulary words (2R-3L): science, hypothesis, theory, observation, data, inference, phenomena, independent variable, dependent variable, constant, control, qualitative, quantitative , law, evaluate, analyze,(due Tuesday). Instructor: Ms. Tate Phone: 469-272-2050 ext. 7670 Email: carlene.tate@chisd.net Tutoring: Tuesday and Thursday 7:45-8:05 am Description Biology is the study of living organisms. This course includes the study of plants, animals, ecology, and the fundamental concepts of life science. The students will participate in laboratory activities and science fair that are required by the district. The biology curriculum is based on the required TEKS for science. Biology is a required course as described by the Texas Education Agency Department Mission: The mission of the NGC science department is to provide the students with the ability to make informed decisions using critical thinking and scientific problem solving skills. Learning Outcomes The student will be able to define and correctly use scientific terminology in regard to biological organisms and processes, synthesize information, think critically and solve critical thinking problems, work well independently and in small groups, show self direction and motivation, contribute to group work, assess personal needs in regard to study time and methods, and accept personal responsibility for the learning process. Grading Scale There will be a minimum of 12 grades per six weeks, including two major grades. Your assignments and your grades will be posted on Family Access weekly (Family Access is found on www.chisd.com). Test/ Project = 40% Quiz = 20% Homework = 10% Daily Work/Lab = 30% Labs The student for at least 40% of instructional time will conduct laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student will be held accountable for completing a minimum of 2 lab reports a six weeks using the following format. Objective Introduction Hypothesis Safety Materials Experimental procedures Results/Data Conclusion Projects Every student is required to participate in the district science fair. It begins very early this year, so start thinking about possible topics. Students will also be required to read “Panic in Level 4”. Students can also choose another book from an approved list and complete a book report for extra credit at your teachers’ discretion. Current scientific events will be due every six weeks. Students are responsible for choosing an article based on the topics being presented in class for the six weeks. By the end of the year, six article reviews will be completed and submitted. Additional information and instructions will be provided on each project. Course Outline by Six Weeks 1st Six Weeks Science Safety and EvidenceBased Thinking Ecology Biochemistry 4th Six Weeks Genetic and Heredity Evolution 2nd Six Weeks Biochemistry (cont) Cells Cellular process: photosynthesis and cellular respiration 5th Six Weeks Evolution (cont) Classification Viruses and Bacteria Plant structures and Adaptations 3rd Six Weeks Cellular process: Cell cycle Cellular process: Protein synthesis 6th Six Weeks Body Systems Experimental Designs Student Expectations My Promise to Students I will be in class on time, well organized and ready to teach My Expectations of Students Students will be in class on time, well organized and ready to learn. I will never yell at you or belittle you in class I will give careful thought to class instructions and will make them as clear as I can. Students will not yell or belittle others in class Students will listen to and follow instruction the first time they are given. I will review every assignment you turn in. Students will be expected to turn in every assignment. I will check for understanding and will find more than one way to explain concepts. I will schedule time before and after school to tutor students. Students will participate in class discussions, help explain concepts to others and will ask questions when they do not understand something. I will not take things from your locker or you home. Students will not take things from the teachers’ desk, cabinet, or shelves. I will always be honest with students, parents and administrators Students will be honest with teachers, parents, and administrators. I will manage time effectively and will teach students responsibly. Students will follow procedures and share responsibility for making good use of class time. Classroom Procedures •Dress Code ( shirts, pants, id badge, hair, etc.) should be in compliance before your enter. •Immediately come in and check the board/projector for directions and review the daily agenda. Leave backpacks in their designated area. ( along side the wall cabinets) •Have homework, composition book, and pencils/pens out on your desk. Sharpen pencils before class begins. •Sit in your assigned seat. (unless other directives are given). •Wait for further instructions. Specifics: • Start Cleaning the room 5 min prior to the sound of the bell, pack up your belongings 1 min prior to the bell., push chairs up and line up at the door. • When you are tardy sign the tardy log with the date, class period, time and your name • If you would like to leave the room for ANY reason, raise your hand and wait for the teacher to address you. • Raise your hand if you would like to move from your seat ( for trash, pencil sharpening, blowing of the nose, etc.) • Do NOT hang out around the sinks and the lab tables. • NEVER talk while your instructor is speaking. Academic Dishonesty Academic honesty is expected, and integrity is valued at Cedar Hill Ninth Grade Center. Scholastic dishonesty is a violation of the Code of Student Conduct. Scholastic dishonesty includes, but is not limited to, cheating on a test, plagiarism, and collusion. Students who Makeup Work If the student is absent, it is the student’s responsibility to get the notes and work missed while absent. The work will be placed in a class period folder and picked up by the student the day they return from being absent. Any student absent will have 2 days to complete and turn in any classwork/homework or quiz that was missed. It is also recommended that the next available tutorial session be attended. It is STRONGLY advised that any test or quiz missed be made up during tutorials offered on Tuesday or Thursday afternoon. Late Work Assignments are written on the board daily and should be copied on the student’s assignment sheet. All assignments must be submitted to the teacher for confirmation of student knowledge and skills. Students should do their own work and neatly show all scientific processes if necessary. Late homework must be turned in within 2 days from the original due date for a maximum grade of a 70 or the student will receive a 0. If you do not have your homework, a missing assignment report MUST be filled out ( by you and your parent/guardian) and returned within the 2 (two) days allotted with the homework assignment. Homework assignments are ALWAYS posted on the webpage as well as your week at a glance from the PowerPoint. Key Understandings •To be knowledgeable in safe practices during field and laboratory investigation. •What are the characteristic of prokaryotic and eukaryotic cells? •How is evolution defined in genetic terms? •How are evolutionary relationships important in classification? •How do the body systems maintain homeostasis in living organisms? Objectives •Students will conduct laboratory and field investigations using the proper methods and equipment, while applying proper scientific reasoning. •They will know the structures of a cell and all the different functions of the cell. •Students will understand the mechanism of genetics and how they can lead to the process of evolution. •They will understand the classification system used and how to categorize the different organism in hierarchal levels. •The students will understand the different biological system and interdependent and how they maintain Cheer One Cedar Hill Longhorns The best on the scene. We're here to win, And dominate your team. Freshman are here We come and attack, Red, black, and white Well put up a fight. C. H. H. S We won't be undone. Watch out for us, There's much more to come Go freshman! Cheer two Cedar hill.....a step above the rest. The red, black and white, We'll put you to the test. Tip of the top, Cream of the crop The team you wan to be That's right, we said it The longhorn dynasty. Yeah! Thursday 8-29-13 Students will demonstrate an understanding of laboratory safety ( precautions and procedures). 1. 2. 3. 4. Engage: http://www.youtube.com/watch?v=xJG0ir9nDtc Journal Set Up (20 min) Lab Safety Review (15 min) ( PowerPoint) Lab Video: List at least 15 incorrect procedures being displayed while viewing the lab safety skit. ( 10 min) 5. Clean Up ( 5 min prior to sound) Reminder: Homework ( contract and contact sheet) due tomorrow. Safety Symbols and vocabulary due next Tuesday. Study Schedule : Daily for at least 25-30 minutes ( notes, vocab, handouts) Lab Safety Thursday 8-29-13 General Safety Rules 1. Listen to or read instructions carefully before attempting to do anything. 2. Wear safety goggles to protect your eyes from chemicals, heated materials, or things that might be able to shatter. 3. Notify your teacher if any spills or accidents occur. General Safety Rules 4. After handling chemicals, always wash your hands with soap and water. 5. During lab work, keep your hands away from your face. 6. Tie back long hair. General Safety Rules 7. Roll up loose sleeves. 8. Know the location of the fire extinguisher, fire blanket, eyewash station, and first aid kit. 9. Keep your work area uncluttered. Take to the lab station only what is necessary. General Safety Rules 10. It is suggested that you wear glasses rather than contact lenses. 11. Never put anything into your mouth during a lab experiment. 12. Clean up your lab area at the conclusion of the laboratory period. 13. Never “horse around” or play practical jokes in the laboratory. Glassware Safety 1. 2. 3. Chipped or cracked glassware should not be used. Show it to the teacher. Broken glassware should not be disposed of in a classroom trashcan. There is a special glass disposal container for it. When pouring liquids into glassware, make sure the container you are pouring into is resting on a table at least a hands breadth from the edge. Glassware Safety 4. Pour down a glass stirring rod to prevent liquids from splattering. 5. If a piece of glassware gets broken, do not try to clean it up by yourself. Notify the teacher. 6. When inserting glass tubing into a rubber stopper, apply a lubricant like glycerin to the glass and use a twisting motion. Glassware Safety 7. Do not place hot glassware in water. Rapid cooling may make it shatter. Chemical Safety 1. Wear protective goggles and a lab apron whenever heating or pouring hazardous chemicals. 2. Never mix chemicals together unless you are told to do so (and then only in the manner specified). 3. Always refer to your Material Safety Data Sheet (MSDS) for chemical reactivity, flammaility, handling, and storing of the substance in a proper and safe manner. 4. Never taste any chemicals (you should never taste anything in the lab). Chemical Safety 5. If you need to smell the odor of a chemical, waft the fumes toward your nose with one hand. Do not put your nose over the container and inhale the fumes. 6. Never pour water into a concentrated acid. Acid should be poured slowly into water. A&W=Acid to Water Chemical Safety 7. Always use a fume hood to provide ventilation. When exposed to hazardous or noxious fumes, vapors or dust. 8. Follow the instructions of your teacher when disposing of all chemicals. 9. Wash your hands after handling hazardous chemicals. Electrical Safety 1. 2. 3. Lay electrical cords where no one can trip on them or get caught in them. Be sure your hands and your lab area are dry before using electrical equipment. Never poke anything into electrical outlets. Electrical Safety 4. 5. Unplug cords by pulling the plug and not the cord. Unplug all electrical equipment at the end of the lab period. Heating Safety 1. 2. 3. Let burners and hotplates cool down before touching them. Test to see if they are cool enough by bringing the back of your hand close to them. Use tongs and/or protective gloves to handle hot objects. Never reach across an open flame or burner. Heating Safety 4. 5. 6. The only type of glassware that may safely be heated is either Kimax or Pyrex. Always point the top ends of test tubes that are being heated away from people. When heating a test tube, move it around slowly over the flame to distribute the heat evenly. Heating Safety 7. 8. Only glassware that is thoroughly dry should be heated. Heat glassware by placing it on a wire gauze platform on a ring stand. Do not hold it in your hand. Heating Safety 9. When lighting a burner, wait until the striker is in place before you turn on the gas. 10. The amount of air can be adjusted by the air supply valve below the tube of the burner. This regulates the flame temperature and color. 11. Never leave a burner or hotplate unattended. First Aid Injury: Burns What To Do: Immediately flush with cold water until burning sensation is lessened. Notify your instructor immediately! Notify your instructor immediately! First Aid Injury: Cuts, bruises What To Do: Do not touch an open wound without safety gloves. Pressing directly on minor cuts will stop bleeding in a few minutes. Apply cold compress to bruises to reduce swelling. First Aid Injury: To Do: Fainting Provide fresh air and have the person recline so that their head is lower than the rest of their body. Notify your instructor immediately! First Aid Injury: Eyes What To Do: Flush eyes immediately with plenty of water for several minutes. If a foreign object is lodged in the eye, do not allow the eye to be rubbed. Notify your instructor immediately! First Aid Injury: Poisoning What To Do: Find out what substance was responsible for the poisoning and alert the teacher immediately. Notify your instructor immediately! First Aid Injury: Spills on the skin What To Do: Flush with large quantities of water. For acid spills, apply baking soda solution. For base spills, apply vinegar or boric acid. Notify your instructor immediately! First Aid Injury: Electrical shock What To Do: Shut off the current at the source. Remove wire with rubber gloves. Alert the teacher immediately. Notify your instructor immediately! Safety Symbols Provides a visual hazardous warning on buildings, door, classrooms, and chemical bottles. Interpret this warning label. How to read a chemical label All chemical containers are required to have a label with the complete chemical name. The hazards associated with the material must also be listed on the label. Where would we find the flammability of the chemical? Where would we find any special hazard instructions? Where would we find health risks of the chemical? What is the name of the chemical? Where would we find the reactivity of the chemical? Material Safety Data Sheets (MSDS) Used to provide individuals and emergency personnel with procedures for handling or working with that substance in a safe manner, and includes information Includes: physical data (melting point, boiling point, flash point, etc.), toxicity, health effects, first aid, reactivity, storage, disposal, protective equipment, and spill-handling procedures. Safety Symbols You will see these symbols in laboratory activities. Additional symbols Recyclin g Toxic Eyewash Sign or Symbol Use this sign to indicate the location of an eyewash station. Safety Shower Sign or Symbol This is the sign or symbol for a safety shower. First Aid Sign Use this symbol to identify the location of a first aid station. Defibrillator Sign This sign indicates the location of a defibrillator or AED. Fire Blanket Safety Sign This safety sign indicates the location of a fire blanket. Radiation Symbol Unofficial Radiation Symbol Biohazard This is the safety symbol for a biohazard. Radioactive Symbol This trefoil is the hazard symbol for radioactive material. Ionizing Radiation Symbol This is the ionizing radiation warning symbol. Recycling Symbol Universal recycling symbol or logo. Skull and Crossbones Skull and Crossbones Toxic This is the hazard symbol for toxic substances Eyewash Sign or Symbol Fire Blanket Safety Sign Ionizing Radiation Symbol Safety Shower Sign or Symbol First Aid Sign Defibrillator Sign Biohazard Radioactive Symbol Radiation Symbol Recycling Symbol Skull and Crossbones Toxic Harmful or Irritant This is the hazard symbol for an irritant or the general symbol for a potentially harmful chemical. Flammable This is the hazard symbol for flammable substances. Explosives This is the hazard symbol for explosives or an explosion hazard. Oxidizing This is the hazard symbol for oxidizing substances. Corrosive This is the hazard symbol indicating corrosive materials. Environmental Hazard This is the safety sign indicating an environmental hazard. Respiratory Protection Sign This sign tells you respiratory protection is required. Gloves Required Symbol This sign means you need to wear gloves or other hand protection. Eye or Face Protection Symbol This symbol indicates mandatory eye or face protection. Protective Clothing Sign This symbol indicates mandatory use of protective clothing. Protective Footwear Sign This sign indicates mandatory use of protective footwear. Eye Protection Required Sign This sign or symbol means that proper eye protection must be worn. Harmful or Irritant Corrosive Eye or Face Protection Symbol Flammable Explosives Oxidizing Environmenta l Hazard Respiratory Protection Sign Gloves Required Symbol Protective Clothing Sign Protective Footwear Sign Eye Protection Prohibition Symbol This is a generic prohibition sign or symbol. Nonpotable Water Symbol Symbol for nonpotable water or no drinking water. Do Not Touch Sign This is a Do Not Touch or Do Not Grip sign. No Open Flames Sign This sign prohibits flames and smoking. Do Not Eat or Drink Sign This is the prohibition sign for No Eating or Drinking. Do Not Enter Sign This sign prohibits entry or unauthorized entry. Reactive Material Symbol Symbol for a highly reactive material. Carcinogen Hazard Symbol UN symbol for carcinogens and mutagens. Low Temperature Warning Symbol This symbol indicates the presence of a low temperature or cryogenic hazard. Hot Surface Warning Symbol This is a warning symbol indicating a hot surface. Magnetic Field Symbol This is the warning symbol indicating the presence of a magnetic field. Optical Radiation Symbol This symbol indicates the presence of an optical radiation hazard. Prohibition Symbol Do Not Eat or Drink Sign Low Temperature Warning Symbol Nonpotable Water Symbol Do Not Touch Sign No Open Flames Sign Do Not Enter Sign Reactive Material Symbol Carcinogen Hazard Symbol Hot Surface Warning Symbol Magnetic Field Symbol Optical Radiation Symbol Friday 8-30-13 Students will continue to demonstrate an understanding of lab safety; students will be able to identify lab equipment and understand their functions; students will understand the process of the scientific method. Due Today: Safety Contract and Parent/Student Info Sheet Homework: Unit One Vocabulary (2R-3L); Safety Symbols ( 4L); study safety rules, symbol meanings, lab equipment and unit one vocabulary ( due Tuesday) quiz Thursday, Sept. 5th . Agenda: 1. Lab Equipment Race- ( 3 min)- hand in to teacher 2. Scientific Method ( 7R); Scientific Processes (8L) 3. Finish Lab Skit in journals (7L) 4. Practice Questions – Work with your desk-mate to answer the 12 practice questions over lab safety. You will have (20 minutes) to complete this task. ( 8R)**** Scientific Method It is a process that is used to find answers to questions about the world around us. There are several versions of the scientific method but all have the same basic steps. Scientific Method 1. 2. 3. 4. 5. 6. Observation Hypothesis Test with an experiment Analyze results or data Draw conclusion Communicate results 1. Observation Ask a question A phenomena unexplained occurrence that happens in the natural world leading you to a question. -based on observations and information gained through previous research or knowledge. -must be will defined, measureable, and controllable. Practice Observing What are 3 things you can observe in the picture? Example: I observe grass and trees on the mountain tops 2. Hypothesis What do you think will happen? Speculative guess that has yet to be tested. A prediction is an assumption one makes based off what they have observed. Stated in a If Then statement. Must be testable. Always ask yourself. Is this testable? Practice Predicting Predict 3 things that might happen in theExample: picture . I predict the grass and trees will expand to the other mountain tops if they get enough rain. hypothesis Using one of your observations + predictions forms a testable hypothesis. Example: If there is more than 20 inches of rain a year then an additional 20 feet of grass and trees will cover the mountain tops. Hypothesis Vs. Theory A hypothesis attempts to answer questions by putting forth a plausible explanation that has yet to be rigorously tested. A theory, on the other hand, has already undergone extensive testing by various scientists and is generally accepted as being an accurate explanation of an observation but not proven. (example: Big Bang Theory) 3. Test with a experiment How will you test your hypothesis. Develop a procedure for experimentation incorporate safety procedure -Test with variables 1. Independent variable-what you control (The If) 2. Dependent variable- is what will be measured. What the investigator thinks will be affected during the experiment (The Then). 3. Controlled variable-extraneous factors (unrelated), possibly affecting the experiment must be kept constant. independent and dependent variable in a hypothesis Example: If there is more than 20 inches of rain then an additional 20 feet of grass and trees will cover the mountain tops. Identify the independent variable. If there is more than 20 inches of rain Identify the dependent variable. Then an additional 20 feet of mountain tops will be covered with gra It is being measured Identify a control. cover a portion of the mountain that gets no rain. when testing an experiment. 1. Need multiple samples 2. Combine data from multiple trials 3. Peer review (Can someone else follow your procedures and get same results. 4. Analyze results/Data: Data can be qualitative or quantitative Qualitative-Deals with descriptions. Data that can be observed but not measured. Qualitative → Quality Some friendly red ants Quantitative-Deals with numbers. Data which can be measured. Quantitative → Quantity 12 red ants Using the illustration practice identifying qualitative and quantitative Practice quantitative vs. qualitative (example the picture is Quantitative-list three 10in x 14 in Qualitative-list three (example the picture has blues, greens, and brown colors) Analyze results and Data 3 Types of graphs 1. Bar graph-useful in comparing information gathered by counting 2. Pie graph-Shows a fixed quantity. Is broken down into parts. Also show a percent(%) that will equal 100. -How to calculate percent. Take the total # divided by amount multiplied by 100 (class practice). 3. Line graph-Show a relationship between variables over time (independent vs. dependent) Why Analyze results/Data To see if the result of the M experiment support or D refute the hypothesis. I R What can you infer (inference) from the data Y L E MIX manipulative independent x-axis S DRY dependent responsive y-axis 20 18 16 14 12 10 8 6 4 2 0 growth of grass and trees 0 inches 10 inches MIX Inference What do you think the picture or graph is based on using your observations and what you already know. -My observation: a lot of green grass and trees. -What I already know: trees and grass require water to grow and stay green. Example: I infer that this area gets an abundance of rain because of the bright green vegetation Graphing Practice 1. Identify the 3 graphs as pie, line, or bar. 2. Identify the independent and dependent variable on the line and bar graphs. 3. What percentage of Americans enjoy romance movies? 4. What can you infer from the line graph? 5. Draw conclusions Did the data support the hypothesis? If there is more than 20 inches of rain then an additional 10 miles of grass and trees will cover the mountain tops. If yes move forward If no must reconstruct hypothesis. M 20 18 D I R L Y E S 16 14 12 10 8 6 4 2 0 growth of grass and trees 0 inches 10 inches MIX Does the graph support my hypoth YES 6. Communicate Results Presented in the form of a lab report. Summarizes the important parts of your experiment and the results. Share your results with others. So others can replicate the experiment and expand on it. Lab Report Template Title: * a brief, concise, yet descriptive title Statement of the Problem: * What question(s) are you trying to answer? * Include any preliminary observations or background information about the subject Hypothesis: * Write a possible solution for the problem. * Make sure this possible solution is a complete sentence. * Make sure the statement is testable. Materials: * Make a list of ALL items used in the lab. Procedure: * Write a paragraph (complete sentences) which explains what you did in the lab. * Your procedure should be written so that anyone else could repeat the experiment. Results (Data): * This section should include any data tables, observations, or additional notes you make during the lab. * You may attach a separate sheet(s) if necessary. * All tables, graphs and charts should be labeled appropriately Conclusions: * Accept or reject your hypothesis. * EXPLAIN why you accepted or rejected your hypothesis using data from the lab. * Include a summary of the data - averages, highest, lowest..etc to help the reader understand your results * List one thing you learned and describe how it applies to a real-life situation. *Discuss possible errors that could have occurred in the collection of the data (experimental errors)