CHM 4412 Chapter 12 - University of Illinois at Urbana
advertisement

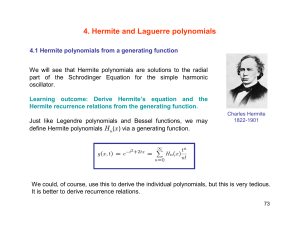
Lecture 10 Harmonic oscillator (c) So Hirata, Department of Chemistry, University of Illinois at Urbana-Champaign. This material has been developed and made available online by work supported jointly by University of Illinois, the National Science Foundation under Grant CHE-1118616 (CAREER), and the Camille & Henry Dreyfus Foundation, Inc. through the Camille Dreyfus Teacher-Scholar program. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the sponsoring agencies. Vibrational motion Quantum-mechanical harmonic oscillator serves as the basic model for molecular vibrations. Its solutions are characterized by (a) uniform energy separation, (b) zeropoint energy, (c) larger probability at turning points. We introduce the concept of orthogonal polynomials. Here, we encounter the first set: Hermite polynomials. Differential equations Differential equations 18th-19th century mathematical geniuses Custom-made solutions: Orthogonal polynomials Know, verify, and use the solutions (thankfully) We Vibrational motion Quantum mechanical version of a “spring and mass” problem. The mass feels the force proportional to the displacement: F = – kx. The potential energy is a harmonic potential: V = ½kx2 (F = –∂V/∂x). Eigenvalues The Schrödinger equation is: 2 2 æ d 1 2ö çè - 2m dx 2 + 2 kx̂ ÷ø Y = EY If we solve this, we obtain the eigenvalues: æ 1ö k Ev = ç v + ÷ w , w = , v = 0,1,2,… 2ø m è Comparison to classical case Quantum mechanical æ 1ö k Ev = ç v + ÷ w , w = , v = 0,1,2,… 2ø m è Quantum number Classical Amplitude can take any arbitrary value, so energy is not quantized Same frequency k x(t ) A sin(t ), = m 1 2 When the object stretches the spring E kA the most, the total energy is purely potential energy 2 Comparison to the particle in a box The particle in a box Harmonic potential The energy levels Zero-point energy 1 E0 = w 2 Energy separation Molecules never cease to vibrate even at 0 K – otherwise the uncertainty principle would be violated. Ev+1 - Ev = w For molecular vibration, this amounts the energy of photon in the infrared range. This is why many molecules absorb in the infrared and get heated. Quantum in nature Vibrations of CO2 Why is global warming? Quantum in nature Vibrations of H2O Why is water blue? Eigenfunctions The eigenfunctions (wave functions) are Y v (x) = N v ´ H v ( y) ´ e - y 2 /2 æ ö , y= , a =ç ÷ a è mk ø x 2 1/4 Gaussian function Normalization Hermite polynomial We do not have to memorize this precise form but we must remember its overall structure: normalization coefficient x Hermite polynomial x Gaussian function. Orthogonal polynomials The Hermite polynomials are one example of orthogonal polynomials. They are discovered or invented as the solutions of important differential equations. They ensure orthogonality and completeness of eigenfunctions. Hermite Vibrational wave function Laguerre Radial part of atomic wave function Legendre Angular part of atomic wave function The Hermite polynomials Ground-state wave function Since H0 = 1, the ground state wave function is simply a Gaussian function. 0 ( x) N 0 e y2 / 2 N0e æ ö y= , a =ç a è mk ÷ø x 2 x2 / 2 2 1/4 The Hermite polynomials The Hermite polynomials have the following important mathematical properties: They are the solution of the differential equation: 2 d Hv dH v 2y 2vH v 0 2 dy dy They satisfy the recursion relation: H v 1 2 yH v 2vH v 1 0 Orthogonality y /2 y /2 H e H e dy 0, v v 2 v 2 v Verification Second derivatives of a product of two functions 2 2 æ d 1 2ö - y 2 /2 çè - 2m dx 2 + 2 kx ÷ø N v H v ( y)e d ( fg ) df dg g f dx dx dx d 2 ( fg ) d 2 f df dg d 2g 2 g2 f 2 dx dx dx dx dx 2 Verification Let us verify that this is indeed the eigenfunction. Substituting 0 ( x) N 0 e y2 / 2 N0e x2 / 2 2 æ 2ö y= , a =ç a è mk ÷ø x into the Schrödinger equation, we find 2 æ d2 1 2ö - x 2 /2a 2 =? çè - 2m dx 2 + 2 kx ÷ø N 0e 2 2 deax d (ax 2 ) deax ax 2 (2 ax ) e dx dx d (ax 2 ) 2 d 2 e ax d ax 2 ax 2 2 2 ax 2 (2 ax ) e 2 ae 4 a xe dx 2 dx 1/4 Verification Let us verify that this is indeed the eigenfunction. Substituting 0 ( x) N 0 e y2 / 2 N0e x2 / 2 2 æ 2ö y= , a =ç a è mk ÷ø x 1/4 into the Schrödinger equation, we find 2 2 2 æ æ d2 1 2ö x2 1 1 2ö - x 2 /2a 2 - x 2 /2a 2 = ç+ + kx ÷ N 0e 4 2 çè - 2m dx 2 + 2 kx ÷ø N 0e 2m a 2 è 2m a ø 2 2 deax d (ax 2 ) deax ax 2 (2 ax ) e dx dx d (ax 2 ) 2 d 2 e ax d ax 2 ax 2 2 2 ax 2 (2 ax ) e 2 ae 4 a xe dx 2 dx æ 1 2 1 = ç - kx + 2 è 2 1 - x 2 /2a 2 = w N 0e 2 k 1 2ö - x 2 /2a 2 + kx ÷ N 0e m 2 ø Zero point energy Exercise Calculate the mean displacement of the oscillator when it is in a quantum state v. Hint: Use the recursion relation to expose vanishing integrals H v 1 2 yH v 2vH v 1 0 Exercise x x v dx N * v N 2 v (H ve y2 / 2 2 v (H ve ) y ( H v e y2 / 2 y2 / 2 ) x( H v e y2 / 2 dx ) dy dy N 2 v H ye N 2 v 1 y2 H v H v 1 vH v 1 e dy 0 2 2 2 2 v y2 dy )dx 1 yH v H v 1 vH v 1 2 Orthogonality Permeation of wave functions Probability in the classically forbidden region gets smaller with increasing v (quantum number). Another correspondence principle result. v Greater probability near the turning points. Again correspondenc e to classical case. v 8 % of probability permeates into the classically forbidden region. Summary The solution of the Schrödinger equation for a harmonic potential or the quantummechanical harmonic oscillator has the properties: The eigenfunctions are a Hermite polynomial times a Gaussian function. The eigenvalues are evenly spaced with the energy separation of ħω and the zero-point energy of ½ħω. The greater probability near the turning points for higher values of v.