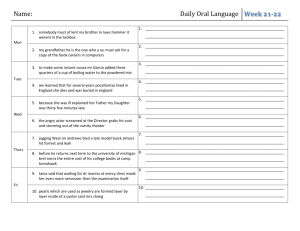
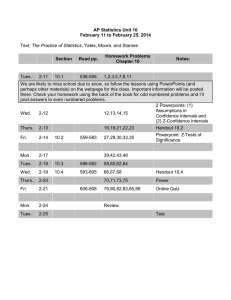
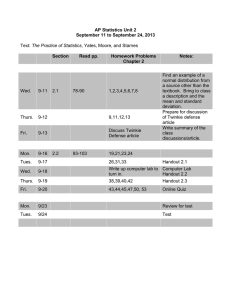
western wyoming community college
advertisement

CHEM 105 ~ Chemistry Foundations ~ 2 credit hours South Dakota State University Spring 2015 Meeting Times and Location: T, Th 11:00 – 11:50 in SAV 0043 (located in the basement of Avera) Instructor: Dr. Nicole Grove Contact Information: Office: Office Hours: Email: SAV 353 M-Th 3:00 – 3:45 (other times by appointment) nicole.grove@sdstate.edu Course Description: A foundation course designed to prepare students for 100 level chemistry courses. Basic concepts in chemistry including matter, measurement, nomenclature, and stoichiometry will be addressed and mathematical concepts basic to 100 level chemistry courses will be practiced. Prerequisites: None Co-requisite: None Instructional Methods: This is a lecture-based course, which will be delivered by the use of PowerPoint and written instruction using an iPad. Several examples will be given in class and the student will also have the opportunity to practice different problems in lecture. The students will have reading assignments as well as several different types of practice problems outside of class. D2L will be used to post lecture outlines, grades, announcements, and supplemental information regarding this course. Required Materials: Brown, T.L., LeMay, H.E., Bursten, B.E., Murphy, C.J., Woodward, P.M. (2012). Chemistry: The Central Science. New York: Pearson. Any edition is acceptable. Mastering Chemistry: This comes with the book, if purchased in the bookstore. Otherwise, this can be purchased online by itself. This is the online homework system for this class. You can access the site by going to the link below. You will need your student ID#, the course ID#: MCGROVE94939, and an access code OR credit card for payment. Please be sure to enter your student ID# correctly, as this is how Mastering Chemistry and D2L will communicate. http://www.pearsonmylabandmastering.com/northamerica/masteringchemistry/students/getregistered/index.html 1 Attendance: Attendance will not be taken, however, all announcements and material will be presented during the normally scheduled class period. If the student is gone, it is the their responsibility to find out what was missed from a classmate. It is not the instructor’s responsibility to “fill the student in” on what they missed. Academic Integrity: Student Academic Integrity and Appeals: The University has a clear expectation for academic integrity and does not tolerate academic dishonesty. University Policy 2.4 sets forth the definitions of academic dishonesty, which includes but is not limited to, cheating, plagiarism, fabrication, facilitating academic dishonesty, misrepresentation, and other forms of dishonesty relating to academics. The Policy and its Procedures also set forth how charges of academic dishonesty are handled at the University. Academic Dishonesty is strictly proscribed and if found may result in student discipline up to and including dismissal from the University. Academic integrity is the maintenance of professional standards in writing, assessment, behavior, ethics, research and all University activities. Academic dishonesty will not be tolerated. Written and other works as well as other academic activities by students are expected to be the products of their own efforts. Dishonesty, deception, cheating, plagiarism, fabrication, complicity, multiple submission and copyright/fair use abuse will not be tolerated. Students are encouraged to work together, however; students must submit their own original work. It is unacceptable for two or more students to collaborate in the creation of an assignment and each present the same material. This will be considered an act of deception. Extracting the work of another, either partially or entirely, without reference citation is plagiarism. Since quotation marks are reserved for profound statements and are unacceptable for use in reports, they may not be used in direct quotations or in lieu of paraphrasing material. In either event, the material must be referenced. Course Goals and Student Learning Outcomes: This course does not meet any IGR or SGR requirements. Students will be introduced to basic chemistry concepts in order to supplement a high school chemistry experience. By the end of this course students should be able to: 1. Identify and explain the basic concepts, terminology and theories of the selected natural sciences. To meet this outcome, students will study and solve problems where they will draw conclusions from lecture material based on sound scientific concepts and principles. Students will evaluate the validity of the data through independent and collaborative work, as well as through written expression. This outcome will be assessed through exams, quizzes, and assignments. The process of lectures, active learning, team-based learning, practice exercises, and reflective questions will be used. Methods of Evaluation: There will be four in-class hour exams, a final exam, which will be cumulative, online homework, and 6 unannounced, in-class quizzes. Each in-class exam will be worth 100 points each. The final exam is comprehensive and will be worth 200 points. 2 Grading Upon grading, exam scores will be posted in D2L for each student. The homework scores will be available for students to view within Mastering Chemistry. If you see an error in the scores posted on D2L or Mastering Chemistry, you have one week from the time the assignment was due, or the score gets posted into D2L to notify the instructor of the mistake. Each homework assignment in Mastering Chemistry will be graded as follows: You will have up to 7 attempts of each homework problem. If you earn at least an 85% on each homework set, you will receive the entire 100% of that homework set grade. Exams: There are no make-up exams. If you will be gone for a university-sponsored event, you must take the exam prior to leaving for the event. No exams will be given after the class has taken the exam. No early final exams will be allowed, so please plan accordingly. The instructor will not accept any late work, and there are no make-up exams or assignments. Please plan accordingly. No extra credit will be given. Assessment Syllabus Quiz Quizzes Journaling/Reflection Questions Homework 4 exams (100 pts each) Final Exam Total: Points 20 60 100 157 400 200 937 Grading Scale: Points 843.3 - 937 749.6 – 843.2 655.9 – 749.5 562.2 – 655.8 561.1 and below % 90.0% 80.0% 70.0% 60.0% <60.0% Grade A B C D F Email Correspondence: All email correspondence will be done with the home university assigned email, NOT through D2L. The student should use their Jacks email when emailing the instructor as other email addresses may be autoforwarded to the spam and quarantine folders without the instructor’s knowledge. Email correspondence will NOT be answered within D2L. Accommodation Assistance: If you are a person with a disability for which you are or may be requesting an accommodation, you should contact the Director of the Office of Disability Services at your home school. At University Center, you contact Jennifer Schelske, Student Services Coordinator at 605-367-8465. For SDSU main campus student, you should 3 contact the Director of the Office of Disability Services for SDSU, which is Nancy Crooks at 605-688-4504 or Nancy.Crooks@sdstate.edu. Tentative Lecture Schedule: Day Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Tues Thurs Wed Date 1/13 1/15 1/20 1/22 1/27 1/29 2/3 2/5 2/10 2/12 2/17 2/19 2/24 2/26 3/3 3/5 3/10 3/12 3/17 3/19 3/24 3/26 3/31 4/2 4/7 4/9 4/14 4/16 4/21 4/23 4/28 4/30 5/6 Chapter Coverage Course Introduction Chapters 1.1-1.3; Classification and Properties of Matter Chapters 1.4-1.5; Measurements and Uncertainty Chapter 1.6; Dimensional Analysis Chapter 1.6; Dimensional Analysis Chapter 1 practice Exam 1 Chapter 2.1-2.5; Atomic Structure and Periodic Table Chapter 2.6-2.7; Formulas and Ions Chapter 2.8-2.9; Ionic nomenclature and Formulas Chapter 2.8-2.9; Molecular nomenclature, Acids and Formulas Fun and Games Exam 2 Chapter 3.1; Balancing Equations Chapter 3.2; Types of Chemical Reactions Chapter 3.3; Formula Weights Chapter 3.4; Avogadro's Number NO CLASS ~ Spring Break NO CLASS ~ Spring Break Chapter 3.5; Empirical Formula from Analysis Chapter 3.6; mass to mass calculations Chapter 3.7; Limiting reagent and percent yield Chapter 3 practice Exam 3 Chapter 4.1; Solution properties Chapter 4.2; Precipitation reactions Chapter 4.3; Neutralization reactions Chapter 4.4; Oxidation-reduction reactions Chapter 4.5; Molarity and Dilution Chapter 4.6; Solution stoichiometry Chapter 4 practice Exam 4 11:30 – 1:30 in SAV 0043 Freedom in Learning: Students are responsible for learning the content of any course of study in which they are enrolled. Under Board of Regents and University policy, student academic performance shall be evaluated solely on an academic basis and students should be free to take reasoned exception to the data or views offered in any course of study. Students who believe that an academic evaluation is unrelated to academic standards, but related instead to judgment of their personal opinion or conduct, should first contact the dean of the college that offers the class to initiate a review of the evaluation. 4 Late Class Statement: All members of the class should make every effort to arrive on time. In the event that I am going to be late, due to circumstances beyond my control, I will, if possible, notify the department and ask that someone be sent to apprise you of the situation. If such notification is not possible, please remain in the class for 15 minutes beyond the scheduled start time. If I have not yet arrived, and if no emissary of the department has informed you otherwise, class will be cancelled and you will be free to leave. Academic Success/Starfish As your professor, my goals are to help you be successful in this course and to make your learning experience as meaningful as possible. For that reason, if you demonstrate any academic performance or behavioral problems that may impede your success, I will communicate with you using Starfish. Starfish is an online student success program that allows me to send various performance updates to you and to those dedicated to supporting your success at SDSU. If you receive a notification in Starfish, please come see me or seek assistance from your advisor, the Student Success Center, or other campus resources. Please make sure to update your Starfish profile at the beginning of each semester (including a photo and up-todate contact information). The Starfish link is located in D2L in the top left corner of your homepage. 5