Strum-Liouville Problems - Artie McFerrin Chemical Engineering
advertisement

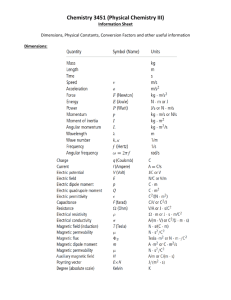
Introduction and Preliminary Concepts Separation of Variables Sturm-Liouville Theory Fourier and Laplace Transforms Linearity, Superposition and Classifications: A partial differential operator (PDO) L is considered linear if the following is satisfied L(u cv) Lu cLv where c is a scalar and u & v are functions For example, L(u cv) 2 (u cv) y (u y 2u ) c(v y 2v) Lu cLv therefore, PDO L is linear. Linearity, Superposition and Classification (3) It follows that for a linear PDO the equation Lu f is homogeneous if f 0 and heterogeneous if f is any other function. The order of a PDE is determined based on the highest order derivative that is in it. For example, the inviscid Burgers’ equation below ut uu x 0 is first-order while the Korteweg-deVries (KdV) equation ut uu x u xxx 0 is third-order due to the additional term. If the linear homogeneous equation Lv 0 is satisfied by u1 ,...un it also follows that u c1u1 ... cnun will satisfy it. Linearity, Superposition and Classification (3) In addition, PDEs can be classified based on the sign of the discriminant B2-AC using the following criteria. parabolic hyperbolic elliptic if B 2 AC 0, if B 2 AC 0, if B 2 AC 0 The heat/diffusion equation, which we will be discussing, is parabolic since, 2u xx ut where is a constant B 2 AC 0 2 ( 2 )(0) 0 Examples of solutions of the equation are shown below. Mathematical techniques for engineers and scientists (2) Image modified from http://home.comcast.net/~sharov/PopEcol/lec12/diffus.html Separation of Variables First order chemical reaction takes place on the catalyst wall of cylindrical container X=R Find out the concentration change of A inside the container Set up PDE: X=0 Catalyst wall r=kA D*Axx=At, or D*∂2A/∂x2 =∂A/∂t A-concentration of A D-diffusivity *from Chemical Engineering Kinetics (6) First order chemical reaction takes plane on the catalyst wall Find out the concentration change of A inside the cylindrical contatiner X=R Boundary and initial conditions: X=0 Catalyst wall r=kA I.C t=0, A=A0 B.C1 x=0, ∂A/∂x │x=0 =0, by symmetry B.C2 x=R, D*∂A/∂x │x=R =k*A, diffusion =reaction at wall Run dimensionless: u=A/A0, s=x/R, τ=t/tc (=tD/R2) X=R Then we get: tc*D/R2∂2u/∂s2 =∂u/∂τ, let tc*D/R2=1, then tc=R2/D X=0 Catalyst wall r=kA BCs: ∂u/∂s =0 at s=0 ∂u/∂s =kR/D*u=φu, φ=kR/D at s=1 Assume: u=F(s)*G(τ) G(τ)*dF2/ds2=F(s)*dG(τ)/ds, 1/F(s)*dF2/ds2=1/G(τ)*dG(τ)/d s=-λn2 X=R Solve 1/G(τ)*dG(τ)/ds=-λn2 X=0 Catalyst wall r=kA we get: dG(τ)=exp(-λn2τ) For 1/F(s)*dF2/ds2=-λn2 assume F(s)=∑(Ansinλns+ Bncosλns) Use BC1: ∂u/∂s =0 at s=0 ∂u/∂s=G(τ)*dF/ds=0 X=R ∑(Anλncosλn*0- Bnλnsinλn*0)=0 An=0 F(s)= ∑Bncosλns X=0 Catalyst wall r=kA Use BC2: ∂u/∂s =φu at s=1 -Bnλnsinλn=φBncosλn -λntanλn=φ, λncan be solved u(s, τ) =∑exp(-λn2 τ) Bncosλns How to get Bn? X=R τ =0, u(s,0)=1= ∑1*Bncosλns Use orthogonality principle X=0 Catalyst wall r=kA ∫01 *cos(λns)ds =Bn∫01cos2(λns)ds Bn = ∫01 cos(λns)ds/∫01cos2(λns)ds Bn =4sin(λn )/(2λn+sin2λn) 45 40 40 35 35 30 30 Concentration A Concentration A 45 25 20 25 20 15 15 10 10 5 5 0 0 0 2 4 6 time(t) 8 10 0 12 2 4 6 time(t) 8 10 Second order, homogenous, linear differential equation with boundary conditions. Solutions to such problems contain an infinite set of eigenvalues with corresponding eigenfunction solutions Equations from Advanced Engineering Mathematics (9) Problem from Introduction to Differential Equations (7) Problem from Introduction to Differential Equations (7) Problem from Introduction to Differential Equations (7) Problem from Introduction to Differential Equations (7) Problem from Introduction to Differential Equations (7) Problem from Introduction to Differential Equations (7) Many differential equations are in the form of the Sturm-Liouville problem. Sturm-Liouville analysis can help determine integration constants for equations with an infinite number of terms (eigenfunctions). Problem from Heat Conduction (8) The boundary conditions are such that Figure from Heat Conduction (8) Problem from Heat Conduction (8) Problem from Heat Conduction (8) Problem from Heat Conduction (8) Problem from Heat Conduction (8) Problem from Heat Conduction (8) Problem from Heat Conduction (8) Problem from Heat Conduction (8) Problem from Heat Conduction (8) • Other types of problems • • Wave equation, diffusion equation… Other types of boundaries: • Fixed, Periodic, Moving • Handled same as in regular Sturm-Liouville Problem Diffusion Equation: The energy balance over the infinitesimal volume of a rod shown in the figure is as follows kAux kAux x hsx(u u ) vAcu x vAcu x x x x (mcu) t where, m=AΔxσc. Dividing the full equation by AΔxσc and taking the limit as x goes to 0 yields 2u xx ut vux H (u u ) wher e, 2 Variables: c specific heat k hs &H c Ac hsx(u u ) mass density s circumfere nce kAux x v speed of rod u u temperatur e difference x A cross - sectional area h heat trans fer coefficien t k thermal conductivi ty kAux x x x x Heat Conduction and the Heat Equation (5) Image modified from http://www.ceb.cam.ac.uk/pages/finite-element-simulations.html Initial/Boundary Conditions: For all subsequent calculations we assume that diffusion in the rod is one-dimensional and thus set H and and v to zero. 2u xx ut The initial condition is simply a set function for u(x,t) over the length of the rod at t=0 and is written thus, u ( x,0) f ( x) (0 x L ) The boundary condition can fall into one of three categories. Dirichlet boundary condition: u (0, t ) (t ) Meaning that the left end of the bar is kept at a set temperature. Neumann boundary condition: q(0, t ) kux (0, t ) (t ) This boundary condition specifies the heat flow out of the rod. Particularly, setting (t ) 0 means insulating the left end of the rod. Fundamental Solutions (4) Initial/Boundary Conditions (cont`d): Robin boundary condition: Starting with Newton’s Law of Cooling q hA(u s u f ) where, us and uf are temperatures of the solid and fluid respectively. It then follows that, ( L, t ) h[u f u ( L, t )] kux ( L, t ) h[u f u ( L, t )] kux ( L, t ) hu ( L, t ) hu f Heat Conduction and the Heat Equation (5) For non-periodic functions [f(x)] the heat equation can be solved using Fourier t exponential transform and a known Gaussian function x g(x,t) the steps follow….. u ( x ,0 ) f ( x ) f (x ) x Mathematical techniques for engineers and scientists (2) The Fourier exponential transform can be written as F u ( x, t ); x s e isx u ( x, t )dx U ( s, t ) Using the above definition we can write F u xx ( x, t ); x s s 2U ( s, t ) F ut ( x, t ); x s U t ( s, t ) The heat equation can then be rewritten as ut 2 s 2U 0, t 0 IC : U (s,0) G( s) where, G(s) F f ( x); s We can solve for U using the initial condition to yield 2 2 U ( s, t ) G ( s )e s t , s 2 2 2 2 1 Using the inverse Fourier tr ansform (e s e x /( 2 ) ) 2 and the convolutio n theorem ( f ( s ) g ( s ) u( x, t ) f ( x ) g ( )d ) yields f ( ) g ( x t )d where, g ( x, t ) Thus, u ( x, t ) 1 2 (t ) f ( )e ( x ) 2 / 4 2 t 1 2 t e x 2 / 4 2 t d ……(1) Mathematical techniques for engineers and scientists (2) Special Case 1: The simplest solution to the heat equation is for f ( x) constant F by setting a x equation (1) can be rewritten as 2 t u ( x, t ) F 2 t e a 2 2 t da Assuming that all terms except a are constant the function can be integrated to yield u ( x, t ) F F Thus, for the limiting case of f(x) = constant the heat will also equal that constant. Advanced engineering mathematics (9) Special Case 2: In this case, assume the initial condition is as follows Using these initial conditions the heat equation can be solved to yield u ( x, t ) 100 e 4t 0 ( x ) 2 4t d 50 * (1 erf ( x 2 t )) since the integral can then be rewritten as erf ( z ) 2 z e 2 d 0 Beny Neta Presentation (1) From the chart we can see the function “smoothing” out at large values of t. This is due to the behavior of the error function, erf(∞)=1 and erf(~0)=0. Thus, for positive values of x at small times u(x,t) approaches F and at large times u(x,t) approaches F/2. For negative values of x the negative sign can be taken outside of the error function so the value of the error function is subtracted from 1. Thus at small times u(x,t) approaches 0 and at large times u(x,t) approaches F/2. t=10 t=500 t=1000 t=10000 u(x,t) 100 90 80 100000 70 10000000 60 50 40 30 20 10 0 -400 -300 -200 -100 0 100 200 300 x 400 Typical Behavior The following curves are of temperature collected at different times. From the plots a “smoothing” effect can again be seen as the temperature along entire length of the rod approaches an equilibrium value. Images taken from http://en.wikipedia.org/wiki/File:Heatequation_exampleB.gif Special Case 3: Similar to the error function the integral of equation (1) can be rewritten as the kernel ( x ) 2 / 4 2 t K ( x; t ) and the function becomes…. e 2 t f ( ) K ( x; t )d it can be shown that u ( x, t ) K ( x; t )d 1 u ( x, t ) x by setting a 2 t Thus as t 0, K ( x) K to t1 t2 x From the graph you can see that similar “smoothing” process occurs for the Kernel as for the temperature profile. It is also important to note that the Kernel is a response function to an initial temperature function described by the delta function figure modified from: http://mobjectivist.blogspot.com/2010_06_01_archive.html Heat Conduction of a Semi-Infinite Rod (Laplace Transforms) For non-periodic functions [f(x)] t the heat equation u ( x ,0 ) f ( x ) can be solved in f (x ) u (0, t ) the semi-infinite x g (t ) domain using Laplace u u , ( 0 x , t 0) exponential BC : u(0, t ) f (t ), u( x, t ) 0 as x , t 0 transform the IC : u( x,0) 0, (0 x ) steps follow….. 2 xx t Mathematical techniques for engineers and scientists (2) Using the inverse Laplace transform U(x,p) becomes, x2 2 x p e 4 t e 3/ 2 x t 2 xe x /( 4 t ) u ( x, t ) g (t ) 2 (t 3 / 2 ) 2 Then using the convolution theorem 2 t ( f * g )(t ) f ( ) g (t )d 0 u(x,t) becomes, u ( x, t ) x 2 t g (t ) 0 e x 2 / 4 2 3/ 2 d ………(2) Mathematical techniques for engineers and scientists (2) The Laplace exponential transform can be written as L u( x, t ); t p e pt u( x, t )dt U ( x, p) Using the above definition we can write L u xx ( x, t ); t p U xx ( x, p) L ut ( x, t ); t p pU ( x, p) The heat equation can then be rewritten as U xx p 2 U 0, x0 BC : U (0, p ) G ( p ) where G ( p ) L f (t ) U ( x, p) 0 as x We can solve for U using the boundary condition to yield U ( x, p) G( p)e x p , p Mathematical techniques for engineers and scientists (2) Special Case : The simplest solution to the heat equation is for g ( x) cons tan t F By setting a2 x2 4 we can solve for 2 2 x2 2 x 2 2 and d 2 3 da 4 a 4 a Thus equation (2) becomes x 2F or otherwise 2 t e a 2 da 0 u ( x, t ) F * erfc( x 2 t ) Advanced engineering mathematics (9) For F=50 and α=1 u(x,t) is plotted below From the chart we can see a similar “smoothing” process as in the case of the infinite domain at large values of t. Again, erf(∞)=1 and erf(~0)=0. Thus at small times u(x,t) approaches F and at large times u(x,t) approaches 0. u(x,t) 50 40 30 20 10 0 0 x 100 200 300 400 500 600 700 800 900 t=10 t=500 t=1000 t=10000 100000 10000000 References 1.Neta, Beny. "PARTIAL DIFFERENTIAL EQUATIONS MA 3132 LECTURE NOTES." math.nps.navy. Department of Mathematics Naval Postgraduate School, n.d. Web. 20 Oct. 2011. <www.math.nps.navy.mil/~bneta/pde.pdf>. 2.Andrews, Larry C., and Ronald L. Phillips. Mathematical techniques for engineers and scientists. Bellingham, Wash.: SPIE Press, 2003. Print. 3."Linearity, Superposition and Classification." math.unl. N.p., n.d. Web. 21 Oct. 2011. <www.math.unl.edu/~scohn1/8423/class.pdf>. 4."CHAPTER 2: The Diffusion Equation." mth.pdx. N.p., n.d. Web. 21 Oct. 2011. <www.mth.pdx.edu/~marek/mth510pde/notes%202.pdf>. 5."Heat Conduction and the Heat Equation." ncsu. N.p., n.d. Web. 21 Oct. 2011. <www4.ncsu.edu/~rsmith/MA573_F09/Heat_Equation.pdf>. 6.Platnaik. Introduction to Differential Equations. PHI Learning Pvt. Ltd. 7.Jiji, Latif M. Heat Conduction. 3rd Ed. Springer Publishing. 2009. 8.Greenberg, Michael D.. Advanced engineering mathematics. 2nd ed. Upper Saddle River, N.J.: Prentice Hall, 1998. Print.