Honors Chemistry Assignments Chapters 7-8
advertisement

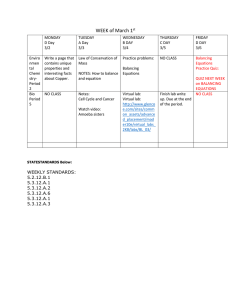
Chemistry Assignments Chapters 7-8: Chemical Reactions GPS: SC2 Students will relate how the Law of Conservation of Matter is used to determine chemical composition in compounds and chemical reactions. a. Identify and balance the following types of chemical equations: • Synthesis • Decomposition • Single Replacement • Double Replacement • Combustion b. Experimentally determine indicators of a chemical reaction specifically precipitation, gas evolution, water production, and changes in energy to the system. Essential Questions: What are the indicators that show a chemical reaction has occurred? What are the characteristics of a chemical reaction? How do you balance an equation? How do you write an equation? What is a precipitate? What are the types of reactions? Vocabulary – Define these: chemical reaction double-displacement reaction balanced chemical equation synthesis reaction precipitate solid molecular equation Date 10/29 11/3 chemical equation single-displacement reaction combustion reactions decomposition reaction strong electrolyte insoluble solid acid Classwork/Homework Schedule Classwork Homework Demo: Water-Wine-Milk-Beer Notes – Chemical Reactions Worksheet – Writing Chemical Equations Ticket out – Writing chemical equations Do Now-Chem Rxn Indicators Review Homework Guided practice – Balancing p. 219 #1-3 & p. 223 #1-4 Prelab Experiment 26 And p. 232 #1-5 reactants products coefficients precipitation soluble base 11/5 11/9 11/11 11/15 Equations Ticket out – Balancing Equations Check and review homework and prelab Exp 26 Experiment 26 Examples of Chemical Reactions Do Now – Balancing Equations Review Homework problems Quiz – Chemical Reactions Do Now- Write and Balance the Equations Review Quiz and Chapter Review Ticket out – Write a question Chemical Reactions Test Article with questions Worksheet – Balancing and Typing Chemical Equations Chapter 7-8 Review handout p. 235 #33-35 Balance and identify type of reaction! Homework All homework will be checked for completion on due date and must be stapled to the assignment sheet and turned in on test day. We will review all in class worksheets prior to the test. If you do not get it stamped prior to class review of the homework you must do the alternate for credit. HW #1 Due 11/3 - p. 219 #1-3 & p. 223 #1-4 _____________ HW#2 Due 11/5 – Prelab Experiment 26 & p. 232 #1-5 ______________ HW#3 Due 11/9 - Worksheet – Balancing and Typing Chemical Equations ____________ HW#4 Due 11/11 - Chapter 7-8 Review handout ____________ HW #5 Due 11/15 - p. 235 #33-35 Balance and identify type of reaction! ___________ Alternative (Makeup) Homework Assignments HW#1 p. 234-235 #1,3,7,8,9,11,13,17,19,21 HW#2 p. 234-235 Write and balance #22-30 HW #3 p. 272 -274 #12-13, 28-29 35-39 all HW #4 p. 236 #37-49 & p. 273 22-23 HW#5 p. 237 #1-12 You must explain why you chose each answer in at least one sentence!