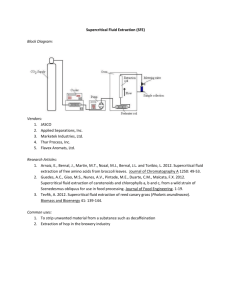
For Solid Samples
advertisement
The Analysis of Real Samples TMHsiung@2014 1/34 1) 2) 3) 4) 5) 6) 7) 8) Steps of quantitative analysis Selecting a method Sampling Preparing a laboratory sample Defining replicate samples by mass or volume measurements Preparing solutions of the samples Eliminating interferences Performing measurements for analyte concentration Computing the results and estimating their reliability TMHsiung@2014 2/34 Choice of analytical method 1) Definition of the Problem • What is the concentration range of the analyte to be determined? What degree of accuracy is desired? What other components are present in the sample? What are the physical and chemical properties of the gross sample? How many samples will be analyzed? • • Chemical abstract/SciFinder Web of Science • • • The Analysis of Standard Samples Using Other Methods Standard Addition to the Sample • • • • 2) Investigating the Literature 3) Testing the Procedure TMHsiung@2014 3/34 Contents in sampling plan – Physically removing the sample from its target population – Preserving and handling the sample – Storing the sample – Preparing the sample for analysis TMHsiung@2014 4/34 For Liquid Samples • • • Examples: beverages, urine, natural waters Sampling tools: pipet, syringe, water sampler etc. Preservation: TMHsiung@2014 5/34 For Gas Samples • Sampling tools: stainless steel canister, Tedlar/Teflon bag, solid sorbent trap, filtering, cryogenic trap etc. • For analyte removing: – Thermal desorption – Extracting with solvent TMHsiung@2014 6/34 For Solid Samples • Sampling tools: Grab sampler(抓取式採樣器)、 Corer(鑽取式採樣器)、Scoops(杓 )/shovel(鏟)、 Thief (套管式採樣刀) • Sample preservation: – Low temperatures – Zero headspace – Adding Inert gas – etc. • Sample preparation: – Reducing the particle size (to reduce sampling variance) – Reducing the sample size (quantity) – Bringing solid samples into solution TMHsiung@2014 7/34 Bringing Solid Samples into Solution – Swirling and heating/Conventional digestion/Wet digestion – Flux/Dry ashing – Microwave digestion TMHsiung@2014 8/34 TMHsiung@2014 9/34 TMHsiung@2014 10/34 Classifying Separation Techniques TMHsiung@2014 11/34 Separations Based on Size 1. Filtration • Gravity/Suction/Pressure • Porous filter • Dissolved phase/particulate phase • Gravimetric analysis TMHsiung@2014 12/34 2. Dialysis A method of separation that uses a semipermeable membrane. * Frequently used to purify proteins, hormones, and enzymes. TMHsiung@2014 13/34 3. Size-exclusion chromatography: • Also called gel permeation or molecular exclusion chromatography • A separation method in which a mixture passes through a bed of porous particles, with smaller particles taking longer to pass through the bed due to their ability to move into the porous structure. TMHsiung@2014 14/34 Separations Based on Mass or Density • If there is a difference in the mass or density of the analyte and interferent, then a separation can use centrifugation. • The sample, as a suspension, is placed in a centrifuge tube and spun at a high angular velocity (high numbers of revolutions per minute, rpm). • Particles of equal density, heavier particles having greater sedimentation rates. • Particles are of equal mass, the highest density have the greatest sedimentation rate. TMHsiung@2014 15/34 Example 1: Separated lysosomes from other components 1. Destroying the cell membranes. 2. Centrifuge at 15,000xg (15,000 times the Earth’s gravity) 20 min. 3. Isolate supernatant from the by decanting 4. Centrifuge the supernatant at 30,000xg for 30 min. 5. Leaving a residue of lysosomes. TMHsiung@2014 16/34 Example 2: Equilibrium–density–gradient centrifugation 1. Establish the density gradients, e.g., solutions of CsCl. (density gradient 1.65 g/cm3 ~ 1.80 g/cm3) 2. Place the the sample, e.g., mixture of proteins, RNA, and DNA, into the centrifuge tube. 3. After centrifugation. 3 Proteins, with a density of less than 1.3 g/cm Protein experience no sedimentation. 3 separates DNA, a density of approximately 1.7 g/cm DNA as a band near the middle of the centrifuge tube. 3 RNA RNA, with a density of greater than 1.8 g/cm collects as a residue at the bottom of the centrifuge tube. TMHsiung@2014 17/34 Separations Based on Complexation Reactions (Masking) Masking: A pseudo-separation method in which a species is prevented from participating in a chemical reaction by binding it with a masking agent to an unreactive complex. TMHsiung@2014 18/34 Cyanide is an appropriate masking agent for Ni2+ because the formation constant for Ni(CN)42– is greater than that for the Ni– EDTA complex. Ni(CN)42– is relatively inert in the presence of EDTA. TMHsiung@2014 19/34 Separations Based on a Change of State Changes in Physical State: 1. Fractional distillation Boiling points versus composition diagram for a near-ideal solution. When the analyte and interferent are miscible liquids, for example, a low-boiling point analyte and a high-boiling point interferent. *The lower boiling point, the higher equilibrium vapor pressure. TMHsiung@2014 20/34 Equipment for a fractional distillation. Temp. TMHsiung@2014 21/34 Changes in Physical State: 2. Recrystallization (fractional crystallization) The solid sample is dissolved solvent, then cool down to promote the growth of large, pure crystal. The purified sample is isolated by filtration. Sample is dried to remove any remaining traces of the solvent. Additional recrystallizations if necessary. TMHsiung@2014 22/34 Changes in Chemical State: 1. Evaporation SiO2 SiO2 4HF SiF4 + 2H2O TMHsiung@2014 23/34 Other types of Changes in Chemical State: 2. Selective precipitation 3. Electrodeposition 4. Ion exchange 5. pH dependent precipitation 6. Complexation/extraction 7. pH dependent complexation/extraction TMHsiung@2014 24/34 Separations Based on a Partitioning Between Phases For a selective partitioning of the analyte or interferent between two immiscible phases. a phase containing a solute is brought into contact with a second phase, the solute partitions itself between the two phases: TMHsiung@2014 25/34 1. Liquid–Liquid Extractions: The density of the two immiscible liquids determines which phase is the upper phase. For aqueous-organic extractions: Density Lower than H2O Density Higher than H2O Diethyl ether Hexane Toluene Chloroform Dichloromethane TMHsiung@2014 26/34 2. Solid-Phase Extractions: TMHsiung@2014 27/34 TMHsiung@2014 28/34 3. Solid-phase microextration (SPME): TMHsiung@2014 29/34 4. Continuous Extractions: (Soxhlet extractor for example) TMHsiung@2014 30/34 5. Microwave extractions: The sample is placed in a sealed digestion vessel along with the liquid extraction phase, and a microwave oven is used to heat the extraction mixture. The extraction to take place at a higher temperature and pressure, thereby reducing the amount of time needed for a quantitative extraction. TMHsiung@2014 31/34 6. Purge and trap: TMHsiung@2014 32/34 7. Supercritical fluids extractions (SFE): Supercritical fluid is a state of matter where a substance is held at a temperature and pressure that exceeds its critical temperature and pressure For example, CO2 at 340 atm and 80oC has the properties between those of a gas and a liquid. Density of supercritical fluid is high than that of gas, allowing good extraction ability from sample. Viscosity of a supercritical fluid is significantly less than that of a liquid solvent, allowing it to pass more readily through particulate samples. TMHsiung@2014 33/34 End of Chapter 35-38 TMHsiung@2014 34/34