How many sig figs are in the following number? 1300
advertisement
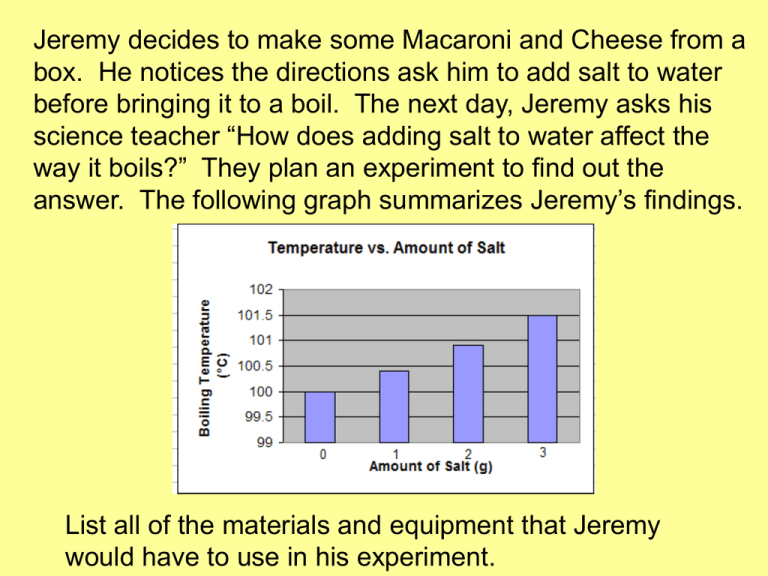
Jeremy decides to make some Macaroni and Cheese from a box. He notices the directions ask him to add salt to water before bringing it to a boil. The next day, Jeremy asks his science teacher “How does adding salt to water affect the way it boils?” They plan an experiment to find out the answer. The following graph summarizes Jeremy’s findings. List all of the materials and equipment that Jeremy would have to use in his experiment. Using Jeremy’s experiment, identify: 1. the independent variable. 2. the dependent variable. 3. the experimental control group. 4. Jeremy’s probable conclusion. Which formula represents a molecular compound? (1) HI (3) KCl (2) KI (4) LiCl Which type of matter is composed of two or more elements that are chemically combined in a fixed proportion? (1) solution (2) compound (3) homogeneous mixture (4) heterogeneous mixture EX 1: Convert 1006051 to scientific notation. 1006051 The natural decimal point is here Decimal will move here 1.006051 x 6 10 6 because the decimal moved 6 times 65 30 2+ Determine the number of protons, neutrons, electrons, and the mass number 30 protons, 35 neutrons, 28 electrons, mass number = 65 EX 2: Convert 0.0052300 to scientific notation. 0 0052300 The natural decimal point is here Decimal will move here 5.2300 x -3 10 Negative because the original number is less than 1 and 3 because the decimal moved 3 times EX: Determine the number of significant figures in a) 4 1 2 b) 4.0 1 c) 40 d) 0.0400 3 e) 4004 4 f) 0.0004 1 Practice EX 1: Determine the number of significant figures in: a) b) c) d) e) f) 2 5.0 10 0.0400 8005 3.00 x 103 1 2 1 3 4 3 EX 2: Convert each of the values into scientific notation while retaining the same number of sig figs a) b) c) d) 60000 20 4004 0.0900 6 x 104 2 x 101 4.004 x 103 9.00 x 10-2 Lets practice together… Round the following numbers to 3 sig figs a) 51.234 51.2 Or 5.12 x 101 1 Or 5.14 x 10 b) 51.38 51.4 51.4 Or 5.14 x 101 c) 51.3587 51,400 Or 5.14 x 104 d) 51,358 -2 0.0506 Or 5.06 x 10 e) 0.050554 YOUR Practice • EX: Round the following numbers to 3 sig figs a) 51.234 51.2 b) 51.38 51.4 c) 51.3587 51.4 d) 51,358 51,400 e) 0.050554 0.0506 f) 5,135,593 5,140,000 Example: 2.8 1 2 3 4 5 6 7 8 9 It is expected that you will always report all sig figs on any laboratory reading! 10 Example: 2.71 1 2 3 4 5 6 7 8 9 It is expected that you will always report all sig figs on any laboratory reading! 10 Which Lewis electron-dot diagram represents calcium oxide? Explain which of the following represent pure substances and which represent mixtures. Practice 1) 22.101 mL – 0.9307 mL = 21.170 mL 2) 2.5 g / 3.42 g = 0.73 g 3) 2.33 cm x 6.085 cm x 2.1 cm = 30. cm3 4) 23.1 in + 4.77 in + 125.39 in + 3.581 in = 156.8 in • EX: 37.24 mL + 10.3 mL = • 27.87 g – 2.2342 g = 47.5 mL 25.64 g Rules for Math Operations with Significant Figures Addition and Subtraction Ex. 37.24mL + 10.3 mL 47.54mL Reported as 47.5mL Rules for Math Operations with Significant Figures Addition and Subtraction Ex. 27.87 g - 21.2342 g 6.6358 g Reported as 6.64g 2. Multiplication and Division • The measurement with the fewest sig figs determines the number of sig figs in the answer • EX: 12.34 cm X 0.23 cm = 2.8 cm2 Practice • 2. Determine the number of g in 0.03455 kg Relationship 1000g = 1kg want Start with what’s given 0.03455 kg 1 1000 g = 34.6 g 1 kg given • Ex. 2 Convert 323 mL to cups Relationships given want 1000 mL = 1L 1L = 1.06 quarts 1 quart = 4 cups Start with what’s given 323 mL 1 1L 1000 mL 1.06 qt. 1L 4 cups 1 qt. = 1.37 cups • Practice 1: Convert 7.005 ft to mm Relationships 1 foot = 12 inches given want 2.54cm = 1 in 100 cm = 1 m 1 m = 1000 mm Start with what’s given 7.005 ft 12 in 1 1 ft 2.54 cm 1 m 1000 mm 1 in 100 cm 1 m = 2135 mm • EX 1: Convert 0.083 km/h to m/s Relationships 1km = 1000m given want 1h = 60 min 1 min = 60 s Start with what’s given 0.083km 1hr 1000m 1 hr 1 min 1 km 60 min 60 s = 2.31 X 10-2 m/s • 1. Convert 3.56 cm/s to ft/h Relationships 2.54 cm = 1 in given want 12 in = 1 ft 60 s = 1 min 1 hr = 60 min Start with what’s given 1 ft 60 s 60 min 3.56 cm 1 in 1s 2.54 cm 12 in 1 min 1 hr = 420 ft/hr • 2. Convert $25.00/feet to cents/cm Relationships $1 = 100 cents given want 2.54 cm = 1 in 12 in = 1 ft Start with what’s given $ 25.00 100 cents 1 ft $1 1 ft 12 in 1 in 2.54 cm = 82.0 cents/cm Determine the number of centimeters in 1.76 kilometers (Show all dimensional analysis.) Determine the number of inches in 697 mm. Note: 2.54 cm = 1 inch (Show all dimensional analysis.) Determine the number of atoms in 5.20 moles of C6H12 . (Show all dimensional analysis.) How many significant figures do the following numbers contain? A) 4,250.00 B) 4040 C) 0.009 D) 325.08 E) 6.02 x 1023 Find the answer to the following using the correct number of significant figures. A) 4,250.38 + 25.1 B) 4040 x 32 Read the following to the correct number of significant figures. Which statement describes a chemical property of bromine? (1) Bromine is soluble in water. (2) Bromine has a reddish-brown color. (3) Bromine combines with aluminum to produce AlBr3. (4) Bromine changes from a liquid to a gas at 332 K and 1 atm. An atom of aluminum in the ground state and an atom of gallium in the ground state have the same (1) mass (2) electronegativity (3) total number of protons (4) total number of valence electrons In an atom of argon-40, the number of protons – (1) equals the number of electrons (2) equals the number of neutrons (3) is less than the number of electrons (4) is greater than the number of electrons Which type of substance can conduct electricity in the liquid phase but not in the solid phase? (1) ionic compound (2) molecular compound (3) metallic element (4) nonmetallic element Why is a molecule of CO2 nonpolar even though the bonds between the carbon atom and the oxygen atoms are polar? (1) The shape of the CO2 molecule is symmetrical. (2) The shape of the CO2 molecule is asymmetrical. (3) The CO2 molecule has a deficiency of electrons. (4) The CO2 molecule has an excess of electrons. The relatively high boiling point of water is due to water having(1) hydrogen bonding (2) metallic bonding (3) nonpolar covalent bonding (4) strong ionic bonding Matter is classified as a (1) substance, only (2) substance or as a mixture of substances (3) homogenous mixture, only (4) homogenous mixture or as a heterogeneous mixture Which substance can not be decomposed by a chemical change? (1) ammonia (3) propanol (2) copper (4) water A beaker contains both alcohol and water. These liquids can be separated by distillation because the liquids have different (1) boiling points (3) particle sizes (2) densities (4) solubilities A sample of an element is malleable and can conduct electricity. This element could be (1) H (3) S (2) He (4) Sn Identify the element in Period 3 of the Periodic Table that reacts with oxygen to form an ionic compound represented by the formula X2O. Based on data collected during a laboratory investigation, a student determined an experimental value of 322 joules per gram for the heat of fusion of H2O. Calculate the student’s percent error. Your response must include a correct numerical setup and the calculated result. Calculate the density of a 129.5gram sample of bronze that has a volume of 14.8 cubic centimeters. Your response must include a correct numerical setup and the calculated result. 8.75 g/cm3 Atomic Structure Questions Answers Determine the number of protons, neutrons, and electrons in an ion of magnesium with a 2+ charge and a mass number of 25. + p = 12 0 n = 13 e = 10 True or False? Elements cannot be created, divided, or destroyed False Elements can be divided (particle accelerators/ atom smashers) Determine the element’s identity: Relative Abundance Element – 28 92.23% Element – 29 4.67% Element – 30 3.10% Silicon The Gold Foil experiment resulted in the discovery of the ______ Nucleus! Europium has two naturallyoccurring isotopes: europium151 with an abundance of 47.82% and europium-153 with an abundance of 52.18%. Determine the average atomic mass for europium. 152.0436 amu Who developed the plum pudding model? JJ Thompson How can you determine the mass number? Protons + neutrons What is fluorine’s most abundant isotope? Fluorine - 19 All atoms of an element are ________ from another element All atoms of an element are different from another element Who discovered the electron? JJ Thompson 3 1 Determine the number of protons, neutrons, and the mass number 1 proton, 2 neutrons, mass number = 3 If 3 protons could be removed from the nucleus of an Nitrogen atom, what nucleus remains? Beryllium 7p – 3p = 4p Determine which element has a mass number of 45 and has 14 neutrons. Gallium Determine the number of protons, neutrons, and electrons in an atom of silicon-29. protons = 14 neutrons = 15 electrons = 14 Write the isotope for the element given that it has 12 protons and 14 neutrons. Show the isotope in both ways. Magnesium – 26 26 12 Mg What part of the atom takes up the most amount of space? electrons What element is used as the standard for atomic mass units? carbon The end of an electron 4 configuration says 4d . What element is represented? What does n represent? Why can’t we have two up spins next to each other? What is the roman numeral of the group 3 found at np ? How many orbitals have electrons in the configuration: 2 2 6 1 1s 2s 2p 3s ***hint***you probably want to write out the orbital notation.



