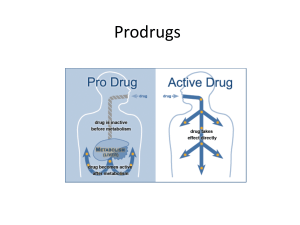
Test Four
advertisement

Chemistry 250. Test Four. November 24, 2014. Name ___________________________________________________ (83 Points) This test is pledged non-disclosure until returned by professor. In Part One, each set of answers also includes e- none of the above. This answer may or may not be needed, but it is included in case I mess up a question. Part One. Two points each – 26 points _____1) Which of the following products is expected when an anhydride is reacted with water (and heat)? a- an anhydride b- an ester c- an acid d- a carboxylate _____2) Which of the following products is formed when an alcohol reacts with a carboxylate (and H +, )? a- an acid b- an anhydride c- an ester d- an amide _____3) Which of the following species is a 2o amine? a- b- c- d- _____4) Which of the following is the product of the aldol reaction of 3-methylbutanal a- b- c- d- _____5) 3-methyl-3-heptanol can be produced by all of the following reactions (all have a last step with H+) EXCEPTa- 2-hexanone with CH3-CH2-MgBr b- 2-pentanone with (CH3)2CH-MgBr c- 3-heptanone with CH3-MgBr d- 2-butanone with CH3-CH2-CH2-CH2-MgBr _____6) The common name of pyruvic acid is a- butanedioic acid b- 2-oxopropanoic acid c- 2,3-dihydroxypropanoic acid d- valeric acid _____7) What is the molecular formula of 2-hydroxy-3,7-dioxo-5-heptenoic acid a- C7H14O5 b- C7H12O5 c- C7H10O5 d- C7H8O5 _____8) Which of the following species is the stronger base a- tributylamine b- butylamine c- dibutylamine d- tetrabutylammonium ion _____9) Which of the following is NOT an ester? a- b- c- d- _____10) Which of the following is the strongest acid? a- b- c- d- _____11) Which of the following is acetylene? a- b- c- d- Part Two. 1) (24 points) Nomenclature a- b- c- d- e- f- g- h- i- j- k- l- 2) (24 points) For each of the following reactions give the organic products. If more than one product is formed, circle the major product. + H+, H2O, → b- + H+, xs 1-butanol → c- + CrO42H+ → d- + SOCl2 → e- + a- - → 2) H+ f- → + ,H+ 3) (9 points) Definitions as we used them Hydrogen bonding Hydrolysis Oxidation