10.1 Sources and Nature of Light
advertisement

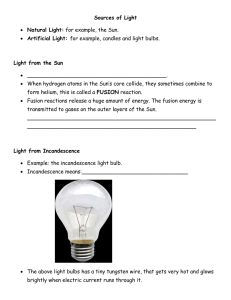
10.1 Sources and Nature of Light Light Emissions • For all light sources, atoms absorb some form of energy. • Excited atoms release energy, often in the form of light. Light from the Sun • H atoms fuse together to form He (fusion reactions). • Energy released from fusion excites gas atoms on outer layers. • Excited atoms emit light. Light from Incandescence • Incandescence = light emitted from material because of high temperature • Incandescent light bulbs have tungsten (W) wires. • 95% of electrical energy released as heat. Light from Electric Discharge • Sodium vapour bulbs (som streetlights) have Na + a small amount of Hg vapour inside. • Electric current moves between electrodes at each end , exciting gas atoms. Fluorescence • Fluorescent bulbs are coated with phosphor & have Hg vapour inside. • Electric current exites Hg atoms, which emit UV light (not visible). • Phosphor absorbes UV & emits visible light (phosphorescence). Efficiency of Fluorescent Lights Uses of Fluorescence Luminescence • Luminescence is light generated without heating an object, e.g., fluorescence. • Phosphorescence is light emitted due to exposure to UV light, that continues to be emitted. Chemiluminescence • Light generated by energy released in a chemical reaction. Bioluminescence • Light produced by living organisms due to chemical reactions in the living cells. The Nature of Light • Light energy travels in electromagnetic waves (involve electric & magnetic fields). • Wavelength = distance from one crest of a wave to the next Electromagnetic Spectrum