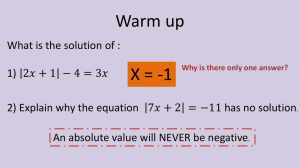Chemical Equations - divaparekh
advertisement

Unit 10: Chemical Equations Chapter Objectives • 1. To learn to write chemical equations • 2. To correctly interpret chemical equations • 3. To balance chemical equations Chapter Objectives • 4. To classify chemical reactions • 5. To predict products of chemical reactions • 6. To write ionic and net ionic equations Chemical Reactions • In a chemical reaction, substances join together to form new substances • The original substances present are called REACTANTS • The new substances formed are called PRODUCTS Discussion of Chemical Reactions • The general form of an equation is: • Reactants Products • The is read as “yields” or “reacts to produce” Discussion of Chemical Reactions • A+BC • Substance “A” and “B” react to produce substance “C” Additional Symbols in Chemical Reactions • + used to separate reactants or products • (s) means chemical is in solid state • (l) means chemical is in liquid state Additional Symbols in Chemical Reactions • (g) means chemical is in gas state • (aq) means chemical is dissolved in water • *See Table 10-1 in book (page 278) Other Symbols • means something is added to the reaction –Usually this is heat Pt • means a catalyst (Pt) is added Skeleton Equations • Skeleton (Formula) Equationthe rough form of an equation • It only shows the framework for the chemical reaction Write Skeleton Equations • Sodium metal reacts with Oxygen gas to form solid Sodium Oxide • Solid sulfur reacts with Fluorine gas to form gaseous Sulfur Hexafluoride when heated • Nitrogen reacts with Hydrogen to form Ammonia (NH3) gas. Heat is required. Review-Write Skeleton Equations • 1. Magnesium metal reacts with Chlorine to form solid Magnesium Chloride. • 2. Aqueous Silver Nitrate reacts with aqueous Sodium Chloride to form solid Silver Chloride and aqueous sodium nitrate Law of Conservation of Mass • The Law of Conservation of Mass states that mass is neither created or destroyed in a chemical reaction • Because of this Law, it is necessary to balance chemical equations Balancing Chemical Equations • In balanced chemical equations, each side of the equation has the same number of atoms of each element • Coefficients are used to balance chemical equations Question • What is the difference between a coefficient and subscript? • Coefficients are written before the formulas • Subscripts are part of the formula • Never use SUBSCRIPTS to balance an equation!! Rules for Balancing Equations • 1. Determine the correct formulas for the reactants and products • 2. Write the formulas for the reactants on the left side of the arrow. Write the formulas for the products on the right side of the arrow Rules Continued • 3. Count the number of atoms of each element present on both sides of the equation • 4. Balance the elements one at a time by placing coefficients in front of the formula. • 5. Check to make sure each atom is balanced Additional Rules • 6. Check to make sure that all coefficients are in the lowest possible ratio • **If no coefficient is written, the coefficient is assumed to be “1” Examples • • • • Balance the following H2 (g) + O2 (g) H2O (l) Na (s) + Br2 (g) NaBr (aq) AgNO3 (aq) + Cu(s) Cu(NO3)2 (aq) + Ag(s) Classwork • Complete Worksheet Review-balance the following • 1. Fe + O2 Fe2O3 • 2. Al2O3 + H2 Al + H2O Quiz Review - Balance • 1. FeCl3 + NaOH Fe(OH)3 + NaCl • 2. CuCl2 + NaI CuI2 + NaCl • 3. H2O2 H2O + O2 QUIZ •1) C6H6 + O2 CO2 + H2O •2) Mg + O2 MgO • 1.QUIZ SolidREVIEW sulfur reacts with gaseous fluorine to produce aqueous sulfur hexafluoride • 2. Magnesium metal reacts with chlorine gas to make solid magnesium chloride Additional Questions • Pb(NO3)2 + 2 NaOH Pb(OH)2 + 2 NaNO3 • How many oxygen atoms are on the reactant side? • How many oxygen atoms are in 2 NaNO3? Balancing Equations Determining Formulas • To Balance Equations, you must remember how to write correct chemical formulas Example • Write the balanced equation for solid aluminum reacting with oxygen gas to form solid aluminum oxide • **Remember that the diatomic elements (Mr. BrINClHOF) appear with a subscript of two when alone Additional Examples • 1. Carbon reacts with Chlorine to form Carbon Tetrachloride • 2. Magnesium metal reacts with solid Zinc (II) Carbonate to form solid Magnesium Carbonate and Zinc metal • 3. Nitrogen gas reacts with Hydrogen gas to form Ammonia (NH3) gas Types of Reactions • There are five general types of reactions: • Synthesis • Decomposition • Single Displacement • Double Displacement • Combustion Synthesis Reactions • Synthesis reactions are also called combination reactions • A synthesis reaction occurs when two substances combine to form a new compound Synthesis Reaction Continued • The general form of a synthesis reaction is: • A + X AX • Substance “AX” is the only substance formed Examples of Synthesis Reactions • 2 Mg (s) + O2 (g) 2 MgO (s) • Fe (s) + Cl2 (g) FeCl2 (s) • U (s) + 3 F2 (g) UF6 (g) Decomposition Reaction • In decomposition reactions, one substance breaks down (decomposes) into two or more simpler substances Decomposition Reactions Cont. • General Form of Decomposition Reaction: • AX A + X Examples of Decomposition Reactions • 2 HgO (s) 2 Hg (l) + O2 (g) • Ca(OH)2 CaO (s) + H2O (g) • H2SO4 (aq) SO3 (g) + H2O (l) Write Correct Balance Equations • 1. The synthesis of KCl • 2. The decomposition of magnesium oxide • 3. The decomposition of hydrogen peroxide (H2O2) into oxygen and water Write Correct Balance Chemical Equations for the following reactions • 1. The synthesis of barium fluoride • 2. The decomposition of Mg(OH)2 into magnesium oxide and water • 3. The decomposition of water Review-Write Balanced Equations • 1. Gaseous hydrogen reacts with gaseous chlorine to form aqueous hydrogen chloride • 2. Carbon monoxide gas reacts with gaseous oxygen to form solid carbon dioxide Write balanced equations • 1. The synthesis of Iron (III) oxide • 2. The decomposition of cobalt (IV) oxide • 3. The decomposition of calcium hydroxide into calcium oxide and water Write Balanced Equations • 1) Na + Cl2 • 2) HgCl2 • 3) Fe(OH)3 Single Replacement Reaction • In a single replacement reaction (also called a displacement reaction), an element reacts with a compound • A + BX AX + B Examples of Single Replacement Reactions • Mg + Zn(NO3)2 Mg(NO3)2 + Zn • Mg + 2 AgNO3 Mg(NO3)2 (aq) + 2 Ag Rules for Single Replacement Reactions • Not all single replacement reactions occur • You can determine if a reaction will occur by knowing the activity series of metals (See Handout) Rules for Single Replacement • The activity series tell you if one metal can replace another metal in a reaction • The Activity Series is ordered • Any metal that is above another metal in the activity series WILL REPLACE the less reactive metal Activity Series • • • • • • • Li K Ca Na Mg Al Zn • • • • • • Fe Pb H* Cu Hg Ag Predict if the following reactions will occur • 1. Fe + H2O • 2. Mg + LiNO3 • 3. Na + AgCl Write balanced equations for the following reactions • • • • 1. 2. 3. 4. Mg + O2 FeCl3 Fe + ZnO Br2 + MgI2 Review • • • • • Predict the products and balance: 1) Mg + O2 2) HCl 3) Na + H2SO4 4) Ag + ZnCl2 Double Displacement Reactions • In a double displacement reaction, two compounds react • The compounds swap elements with each other Double Displacement Cont • Compounds contain a positive and negative part • In a double displacement, the positive parts swap places with each other as do the negative parts Examples • PbCl2 (s) + Li2SO4 (aq) PbSO4 (s) + 2 LiCl (aq) • ZnBr2 (aq) + 2 AgNO3 (aq) Zn(NO3)2 (aq) + 2 AgBr (s) Predict the Products of the following reactions and balance • BaCl2 (aq) + KClO3 (aq) • HCl (aq) + NaOH (aq) • RbBr (aq) + AgCl (aq) Combustion Reactions • In a combustion reaction, a Hydrocarbon (compound containing Hydrogen and Carbon) reacts with Oxygen (O2) • The products are CO2 and H2O Example • 2 C6H6 + 15 O212 CO2 + 6 H2O • *Combustion Reactions commonly require large coefficients Guided Practice • Write the balanced equation for the following combustion reactions: • a. C4H8 • b. C6H12O6 • c. C7H16 Independent Practice- Predict the products for the following reactions • • • • • 1. 2. 3. 4. 5. Hf + N2 (Hf takes a +4 charge) Mg + H2SO4 C2H6 + O2 Pb(NO3)2 + NaI Fe + O2 (Fe takes a +3 charge) Ionic Equations • Most ionic compound dissociate (or break apart) when dissolved in water to form its component ions • For example: NaCl (aq) really looks like Na+(aq) and Cl- (aq) Soluble Vs. Precipitate • Soluble means that the compound breaks down into its ions in water • Ex) NaCl is soluble so it forms Na+ and Cl• Insoluble means that the compound doesn’t break down in water Precipitate Reactions • In double replacement reactions, often one of the product will be insoluble • The insoluble product is referred to as a precipitate Practice • • • • • Determine if soluble or insoluble: A) NaCl B) K2O C) Fe(NO3)3 D) AgCl E) BaS F) Cd(OH)2 G) FeCl3 H) PbCO3 Ionic Equations Continued • To write a Complete Ionic Equation: • Write the aqueous substances as ions (leave any substances in gas, liquids, & solids alone) • Example: • AgNO3 (aq) + NaCl (aq) AgCl (s) + NaNO3 (aq) Writing Ionic Equations • 1. (NH4)2S (aq) + Cd(NO3)2 (aq) NH4NO3 (???) + CdS (???) • 2. Zn(NO3)2 (aq) + (NH4)2S (aq) ZnS (???) + NH4NO3 (???) Spectator Ions • Spectator Ions-Ions that are not directly involved in a reaction • Spectator ions show up on both sides of the equation • Spectator Ions cancel out NET Ionic Equations • Net Ionic Equation-Indicate the particles that actually take part in a reaction • The Net Ionic Equation does NOT include spectator ions • Net Ionic Equations must be balanced according to atoms and charge Write Net Ionic Equations • 1. (NH4)2S (aq) + Cd(NO3)2 (aq) • 2. Zn(NO3)2 (aq) + (NH4)2S (aq)