HW - 3a (ionic bonds & formulas) - Greer Middle College || Building
advertisement
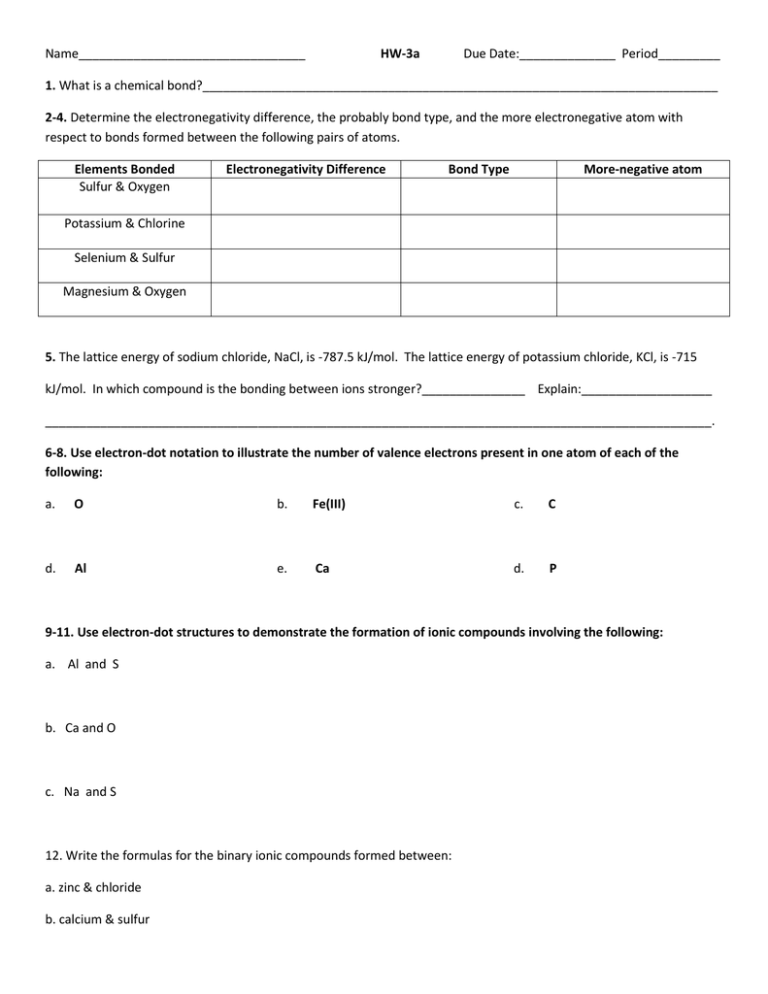
Name_________________________________ HW-3a Due Date:______________ Period_________ 1. What is a chemical bond?___________________________________________________________________________ 2-4. Determine the electronegativity difference, the probably bond type, and the more electronegative atom with respect to bonds formed between the following pairs of atoms. Elements Bonded Sulfur & Oxygen Electronegativity Difference Bond Type More-negative atom Potassium & Chlorine Selenium & Sulfur Magnesium & Oxygen 5. The lattice energy of sodium chloride, NaCl, is -787.5 kJ/mol. The lattice energy of potassium chloride, KCl, is -715 kJ/mol. In which compound is the bonding between ions stronger?_______________ Explain:___________________ _________________________________________________________________________________________________. 6-8. Use electron-dot notation to illustrate the number of valence electrons present in one atom of each of the following: a. O b. Fe(III) c. C d. Al e. Ca d. P 9-11. Use electron-dot structures to demonstrate the formation of ionic compounds involving the following: a. Al and S b. Ca and O c. Na and S 12. Write the formulas for the binary ionic compounds formed between: a. zinc & chloride b. calcium & sulfur 13-14. a. What is an ionic compound?_________________________________________________________________________ b. In what form do most ionic compound occur?___________________________________________________________ c. What is a formula unit?_____________________________________________________________________________ d. In general, what is the relationship of the electronegativities of elements to an ionic bond?______________________ __________________________________________________________________________________________________ 15-16. Write the formulas for and give the names of the compounds formed by the following ions: a. Cr2+ and Fb. Ni2+ & O2c. Fe+3 & SO42- 17. Write the formulas for each of the following: a. strontium oxide c. lithium nitride b. magnesium chloride d. iron(II) oxide e. sodium thiosulfate pentahydrate 18-20. Complete the following charts: Name Chemical Formula Tin nitrate Chemical Formula copper(I) phosphate FePO4 Name Name Chemical Formula N2O4 PBr3 FePO4






![Thermal Stabilities of Delithiated Olivine MPO[subscript 4]](http://s2.studylib.net/store/data/011927215_1-4d4885d3453b1d34b8294c1624ab94af-300x300.png)