CHEM104 QUIZ 2 Spring 2009 Both NaCl and MgO crystallize in the
advertisement

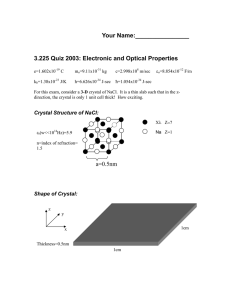
CHEM104 Spring 2009 QUIZ 2 1. Both NaCl and MgO crystallize in the same cubic structure where the anions occupy the sites that define a fcc lattice and the cations occupy all the octahedral sites. The unit cell for NaCl is shown in Fig. 1.9. How will the lattice energy U of NaCl compare to U of MgO? 2. The melting point of NaCl is 801 deg C while the m.p. of NaI is 660 deg C. Assuming that these solids have the same structure, explain the difference in melting points. 3. Dr. B. tells you that she is doing a reaction whose synthesis procedure uses LiCl. However, all she can find in her lab is KCl. She asks you if you think substituting KCl for LiCl will work exactly the same. You pull out your trusty chemistry text and point to the data in Table 14.1 and say “But Dr. B., these numbers show that (what do you say here?)!” 4. The atmospheric pressure on other planets is not necessarily 1 atmosphere as it is on Earth. Imagine a planet whose “normal” atmospheric pressure is 8 atmospheres. Now imagine the behavior of CO2 on this planet: would the term “dry ice” be a misnomer on this planet? Explain why.