Real World Powerpoint
advertisement

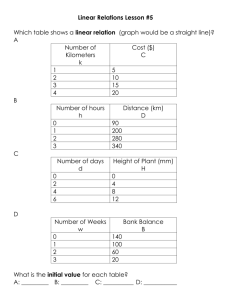
Colleen Justison and Shelby Bleile Atomic Absorption Inductively Coupled Plasma Gun Shot Residue Gun powder burns quickly to produce rapid expansion of gas The three major elements are lead (Pb), barium (Ba), or antimony (Sb). Purpose The purpose of our experiment was to observe the residue left on a person that had been shot at two different distances and angles. We used the AA and ICP to analyze the shirts and determine the amount of Barium and Lead found on them. Procedure Day One Cut t-shirts into “10x10” pieces. Shot a 9 mm Trial One Trial Two Trial Three 6 inches straight 6 inches straight 6 inches straight 6 inches angled 10° 6 inches angled 10° 6 inches angled 10° 12 inches straight 12 inches straight 12 inches straight 12 inches angled 10° 12 inches angled 10° 12 inches angled 10° Cut the shirts in half to give a total of 24 samples. Setup Before & After Procedure Day Two We made a 0.3 M solution of nitric acid from a 16 M solution. Used to soak the t-shirts to remove the solid from the shirts and put it into solution. Made standards of Lead and Barium. 1, 5, 10, 15, 20, 25 ppm. Let the shirts soak in the acid for 24 hours. Procedure Day Three Filtered the shirts. Using vacuum filtration setup we filtered the 24 samples. Prodecure Day four Ran our standards and samples using the AA. found that we needed to make higher concentrated standards because we had residue at higher ppm. The new standards we made were 10, 20, 40, 60, and 80 ppm of Lead and Barium. Procedure Day Four Cont. We ran all 24 samples on both the ICP and AA. We found that the ppm was greater on the AA then on the ICP. Data: Calibration Curves Data: Calibration Curves ICP Data 6 inch Samples T Shirt Samples Barium Conc. (ppm) 6 in. straight Top-T1 1.3359 6 in. straight bottom-T1 0.69785 6 in. straight top- T2 1.2206 6 in. straight bottom-T2 0.81385 6 in. straight top-T3 1.34 6 in. straight botton-T3 2.4792 6 in angle top-T1 0.77045 6 in angle bottom-T1 0.5737 6 in angle top-T2 5.667 6 in angle bottom-T2 3.3847 6 in angle top-T3 1.1268 6 in angle bottom-T3 2.6256 Lead Conc. (ppm) 4.5473 4.1719 4.3365 5.4916 9.3969 26.187 3.0785 3.4544 35.22 26.039 4.0228 10.753 ICP Data 12 inch Samples T Shirt Samples 12 in straight top-T1 12 in straight bottom-T1 12 in straight top-T2 12 in straight bottom-T2 12 in straight top-T3 12 in straight bottom-T3 12 in angled top-T1 12 in angled bottom-T1 12 in angled top-T2 12 in angled bottom-T2 12 in angled top-T3 12 in angled bottom-T3 Barium (ppm) 0.85521 0.4921 0.64699 0.599 0.88931 0.6678 3.4639 2.6992 0.62378 2.355 3.9365 0.50442 Lead (ppm) 14.082 7.4843 2.5193 3.4504 5.299 8.8138 6.398 10.61 5.563 12.137 11.873 3.2191 AA Data- 6 inch Samples Sample 6 in. straight Top-T1 6 in. straight bottom-T1 6 in. straight top- T2 6 in. straight bottom-T2 6 in. straight top-T3 6 in. straight botton-T3 6 in angle top-T1 6 in angle bottom-T1 6 in angle top-T2 6 in angle bottom-T2 6 in angle top-T3 6 in angle bottom-T3 Lead (mg/mL) 18.83 20.87 16.23 22.22 43.17 52.22 20.26 16.84 48.37 36.42 20.02 13.74 Barium (mg/mL) 12.39 15.73 17.67 17.17 20.9 20.84 28.58 28.28 30.83 29.82 32.02 30.68 AA Data-12 inch Samples Sample 12 in straight top-T1 12 in straight bottom-T1 12 in straight top-T2 12 in straight bottom-T2 12 in straight top-T3 12 in straight bottom-T3 12 in angled top-T1 12 in angled bottom-T1 12 in angled top-T2 12 in angled bottom-T2 12 in angled top-T3 12 in angled bottom-T3 Lead (mg/mL) 38.12 35.62 14.98 15.28 24.44 44.93 15.18 13.06 32.95 16.12 14.89 9.417 Barium (mg/mL) 20.9 20.82 23.79 24.7 26.2 26.41 31.86 31.57 32.38 33.51 35.53 34.84 Conclusions The gun shot residue contained more Lead then it did Barium. We saw higher numbers from the AA because it has lower interference. Future Work Use different guns/bullets and test for metals Test for Antimony as well as Lead and Barium Shoot from more angles and distances Use the XRF to observe the samples Acknowledgements Thank you to Chris for making our lives a lot easier during this project and for Andrew for putting up with us all semester. Thank you to the Chemistry department for funding our experiment