Document
advertisement

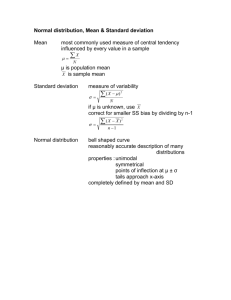
STATISTICAL TESTS AND ERROR ANALYSIS PRECISION AND ACCURACY PRECISION – Reproducibility of the result ACCURACY – Nearness to the “true” value How sure are you that the experimentally obtained value is close to the “true” value? How close is it? Finding errors Experimental error Uncertainty in every experiment (measurement) SYSTEMATIC / DETERMINATE ERROR • Reproducible under the same conditions in the same experiment • Can be detected and corrected for • It is always positive or always negative To detect a systematic error: • Use Standard Reference Materials • Run a blank sample • Use different analytical methods • Participate in “round robin” experiments (different labs and people running the same analysis) RANDOM / INDETERMINATE ERROR • Uncontrolled variables in the measurement • Can be positive or negative • Cannot be corrected for • Random errors are independent of each other Random errors can be reduced by: • Better experiments (equipment, methodology, training of analyst) • Large number of replicate samples Random errors show Gaussian distribution for a large number of replicates Can be described using statistical parameters For a large number of experimental replicates the results approach an ideal smooth curve called the GAUSSIAN or NORMAL DISTRIBUTION CURVE Characterised by: The mean value – x gives the center of the distribution The standard deviation – s measures the width of the distribution The mean or average, x the sum of the measured values (xi) divided by the number of measurements (n) n _ x x i 1 i n The standard deviation, s measures how closely the data are clustered about the mean (i.e. the precision of the data) x x i i n 1 s 2 NOTE: The quantity “n-1” = degrees of freedom Other ways of expressing the precision of the data: • Variance Variance = s2 • Relative standard deviation s RSD x • Percent RSD / coefficient of variation s % RSD 100 x POPULATION DATA For an infinite set of data, n→∞: x → µ and population mean s→σ population std. dev. The experiment that produces a small standard deviation is more precise . Remember, greater precision does not imply greater accuracy. Experimental results are commonly expressed in the form: mean standard deviation _ xs The Gaussian curve equation: 1 y e (x μ ) /2σ σ 2π 2 1 σ 2π 2 = Normalisation factor It guarantees that the area under the curve is unity. Probability of measuring a value in a certain range = area below the graph of that range The Gaussian curve whose area is unity is called a normal error curve. µ = 0 and σ = 1 The standard deviation measures the width of the Gaussian curve. (The larger the value of σ, the broader the curve) Range Percentage of measurements µ ± 1σ 68.3 µ ± 2σ 95.5 µ ± 3σ 99.7 The more times you measure, the more confident you are that your average value is approaching the “true” value. The uncertainty decreases in proportion to 1/ n EXAMPLE Replicate results were obtained for the analysis of lead in blood. Calculate the mean and the standard deviation of this set of data. Replicate [Pb] / ppb 1 752 2 756 3 752 4 751 5 760 xi x _ n s xi 754 x 2 n1 Replicate 1 2 3 [Pb] / ppb 752 756 752 4 5 751 760 3.77 NB DON’T round a std dev. calc until the very end. x 754 s 3.77 754 4 ppb Pb Also: s RSD x 3.77 754 s % RSD 100 x Variance = s2 0.00500 3.77 100 754 3.77 2 14.2 0.500% Lead is readily absorbed through the gastro intestinal tract. In blood, 95% of the lead is in the red blood cells and 5% in the plasma. About 70-90% of the lead assimilated goes into the bones, then liver and kidneys. Lead readily replaces calcium in bones. The symptoms of lead poisoning depend upon many factors, including the magnitude and duration of lead exposure (dose), chemical form (organic is more toxic than inorganic), the age of the individual (children and the unborn are more susceptible) and the overall state of health (Ca, Fe or Zn deficiency enhances the uptake of lead). European Community Environmental Quality Directive – 50 g/L in drinking water Pb – where from? • Motor vehicle emissions • Lead plumbing • Pewter • Lead-based paints • Weathering of Pb minerals World Health Organisation – recommended tolerable intake of Pb per day for an adult – 430 g Food stuffs < 2 mg/kg Pb Next to highways 20-950 mg/kg Pb Near battery works 34-600 mg/kg Pb Metal processing sites 45-2714 mg/kg Pb CONFIDENCE INTERVALS The confidence interval is the expression stating that the true mean, µ, is likely to lie within a certain distance from the measured mean, x. – Student’s t test The confidence interval is given by: _ μx ts n where t is the value of student’s t taken from the table. A ‘t’ test is used to compare sets of measurements. Usually 95% probability is good enough. Example: The mercury content in fish samples were determined as follows: 1.80, 1.58, 1.64, 1.49 ppm Hg. Calculate the 50% and 90% confidence intervals for the mercury content. Find x = 1.63 s = 0.131 _ μx 50% confidence: t= for n-1 = ts n There is a 50% chance that the true mean lies between 1.58 and 1.68 ppm x = 1.63 s = 0.131 1.78 90% confidence: t= for n-1 = 90% 1.68 _ μx ts n 50% 1.63 1.58 μ 1.63 0.15 There is a 90% chance that the true mean lies between 1.48 and 1.78 ppm 1.48 Confidence intervals - experimental uncertainty APPLYING STUDENT’S T: 1) COMPARISON OF MEANS Comparison of a measured result with a ‘known’ (standard) value t calc known value x s n tcalc > ttable at 95% confidence level results are considered to be different the difference is significant! Statistical tests are giving only probabilities. They do not relieve us of the responsibility of interpreting our results! 2) COMPARISON OF REPLICATE MEASUREMENTS For 2 sets of data with number of measurements n1 , n2 and means x1, x2 : t calc x1 x 2 spooled n1n2 n1 n2 Where Spooled = pooled std dev. from both sets of data spooled s12 (n1 1) s22 (n2 1) n1 n2 2 tcalc > ttable at 95% confidence level difference between results is significant. Degrees of freedom = (n1 + n2 – 2) One sample, many measurements 3) COMPARISON OF INDIVIDUAL DIFFERENCES Use two different analytical methods, A and B, to make single measurements on several different samples. Perform t test on individual differences between results: t calc Where d n sd sd 2 (d d ) i n1 Many samples, one measurement Where d = the average difference between methods A and B n = number of pairs of data tcalc > ttable at 95% confidence level difference between results is significant. Example: (di) Are the two methods used comparable? sd t calc (di d )2 n1 d n sd sd 0.12 t calc 1.2 F TEST COMPARISON OF TWO STANDARD DEVIATIONS Fcalc s12 s2 2 Fcalc > Ftable at 95% confidence level the std dev.’s are considered to be different the difference is significant. Q TEST FOR BAD DATA Q calc gap range The range is the total spread of the data. The gap is the difference between the “bad” point and the nearest value. Example: 12.2 12.4 12.5 12.6 Range Gap 12.9 If Qcalc > Qtable discarded questionable point EXAMPLE: The following replicate analyses were obtained when standardising a solution: 0.1067M, 0.1071M, 0.1066M and 0.1050M. One value appears suspect. Determine if it can be ascribed to accidental error at the 90% confidence interval. Arrange in increasing order: 0.1050M Gap Q = Range 0.1066M 0.1067M 0.1071M = 0.7619 BUT these values are very close rather do another analysis to confirm!!! STATISTICS OF SAMPLING A chemical analysis can only be as meaningful as the sample! Sampling – process of collecting a representative sample for analysis OVERALL VARIANCE = ANALYTICAL VARIANCE + SAMPLING VARIANCE 2 2 so sa ss 2 Where does the sampling variance come from? Consider a powder mixture containing nA particles of type A and nB particles type B. Probability of drawing A: nA p = nA+ nB Probability of drawing B: q= nB nA+ nB = 1 - p If n particles are randomly drawn, the expected number of A particles will be np and standard deviation of many drawings will be: σ n npq How much of the sample should be analysed? Std dev. σn npq Where p, q – fractions of each kind of particles present Relative Std Dev. Relative Variance npq σn pq R n n n 2 pq σn R n n 2 nR2 = pq The mass of sample (m) is proportional to number of particles (n) drawn, therefore: Ks = mR2 Where R = RSD as a % and Ks (sampling constant) = mass of sample required to reduce the relative sampling standard deviation to 1% How many samples/replicates to analyse? Rearranging Student’s t equation: Required number of replicate analyses: ts s x n e n t 2 s2s e2 µ = true population mean x = measured mean n = number of samples needed ss2 = variance of the sampling operation e = sought-for uncertainty Since degrees of freedom is not known at this stage, the value of t for n → ∞ is used to estimate n. The process is then repeated a few times until a constant value for n is found. Example: In analysing a lot with random sample variation, there is a sampling deviation of 5%. Assuming negligible error in the analytical procedure, how many samples must be analysed to give 90% confidence that the error in the mean is within 4% of the true value? t 2 s2s n 2 e For 90% confidence: t = n=6 SAMPLE STORAGE Not only is the sampling and sample preparation important, but the sample storage is also critical. The composition of the sample may change with time due to, for example, the following: • reaction with air • reaction with light • absorption of moisture • interaction with the container Glass is a notorious ion exchanger which can alter the concentration of trace ions in solution. Thus plastic (especially Teflon) containers are frequently used. Ensure all containers are clean to prevent contamination. EXAMPLE: (for you to do) Consider a random mixture containing 4.00 g of Na2CO3 ( = 2.532 g/ml) and 96.00 g of K2CO3 ( = 2.428 g/ml) with an approximated uniform spherical radius of 0.075 mm. How many particles of Na2CO3 are in the mixture? And K2CO3? Na2CO3: 4.00 g at 2.532 g/ml m V = = 1.58 ml K2CO3: m V = = 1.58 cm3 96.00 g at 2.428 g/ml VNa2CO3 = 1.58 cm3 VK2CO3 = 39.54 cm3 Particles: r = 0.075 mm = 0.0075 cm nNa2CO3 = 8.94x105 particles nK2CO3 = 2.24x107 particles EXAMPLE: Consider a random mixture containing 4.00 g of Na2CO3 ( = 2.532 g/ml) and 96.00 g of K2CO3 ( = 2.428 g/ml) with an approximated uniform spherical radius of 0.075 mm. What is the expected number of particles in 0.100 g of the mixture? 8.94x102 particles of Na2CO3 and 2.24x104 particles of K2CO3 in a 0.1 g sample EXAMPLE: Calculate the relative standard deviation in the number of particles for each type in the 0.100 g sample of the mixture. R Na2CO3 0.0328 or 3.28% R K2CO3 0.00131 or 0.131%