File
advertisement

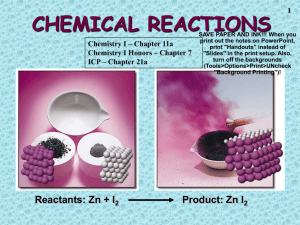
Once you’re in, you’re in. Go to the bathroom/get water before you enter. zero When the bell rings, voice at , working on the Do Now in Do Now Form (flip over the one you have from last week). Pick up daily handouts. Pick up turned back docs. Turn in your MARSHMALLOW ACTIVITY (A LOT ARE MISSING!) TURN IN ANY TEST CORRECTIONS TURN IN ANY TAKE HOME TESTS TURN IN ANY MAKE UP WORK HAVE YOUR HOMEWORK ON YOUR DESK DO NOT TURN IN Do Now 10/15/2013 Find the percent composition of all of the elements in NaOH (Find Na, O, and H) Draw the Lewis structure to determine the molecular geometry, bond angles, and main intermolecular force in NaOH Do Now Review 10/15/2013 Find the percent composition of all of the elements in NaOH (Find Na, O, and H) Draw the Lewis structure to determine the molecular geometry, bond angles, and main intermolecular force in NaOH Tutoring and Makeup Work Tuesday/Thursday 2:30-3:30 Wednesday at Lunch (let me know ahead of time this week) TWO weeks left in the quarter – if your name is on the board , you owe me a quiz or a test!! Zeros Homework Textiles handout Any makeup work / late work Balancing reactions handout** **graded Objectives SWBAT Determine if a chemical reaction has occurred based on precipitate formation, product testing, color change, and temperature change. Write and balance chemical equations Use reference tables to predict products for all types of reactions to show the conservation of mass. Double Replacement Reactions The ions of two compounds exchange places in an aqueous solution to form two new compounds. AX + BY AY + BX One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of solution, or a molecular compound, usually water. Combustion Reactions A substance combines with oxygen, releasing a large amount of energy in the form of light and heat. Reactive elements combine with oxygen P4(s) + 5O2(g) P4O10(s) (This is also a synthesis reaction) The burning of natural gas, wood, gasoline C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) Acid-Base Reactions This is a special kind of double displacement reaction that takes place when an acid and base react with each other. The H+ ion in the acid reacts with the OH- ion in the base, causing the formation of water. Generally, the product of this reaction is some ionic salt and water: HA + BOH ---> H2O + BA One example of an acid-base reaction is the reaction of hydrobromic acid (HBr) with sodium hydroxide: HBr + NaOH ---> NaBr + H2O Exit Ticket 1. MgCl2 + Li2CO3 MgCO3 + 2 LiCl Reaction Type _______________________ 2. P4 + 3 O2 2 P2O3 Reaction Type _______________________ 3. 2 NO2 2 O2 + N2 Reaction Type _______________________ CHEMICAL REACTIONS Reactants: Zn + I2 Product: Zn I2 Chemical Equations Their Job: Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al (s) + 3 O2 (g) ---> 2 Al2O3 (s) The numbers in the front are called stoichiometric coefficients The letters (s), (g), and (l) are the physical states of compounds. Introduction Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. Symbols represent elements, formulas describe compounds, chemical equations describe a chemical reaction Chemical Change definition (two equally good definitions) A chemical reaction is the process by which one or more substances change into one or more new substances whose chemical and physical properties differ from those of the original substances. Chemical Reaction: the process by which one or more substances change to produce one or more different substances. Keys to look for in Chemical Reactions 1. Formation of Gas 2. Color Change 3. Temperature Change 4. Formation of Precipitate 1. A precipitate forms when a substance comes out of a solution and forms a solid Are these chemical reactions? A forest fire destroys acres of land. There is a lot of smoke and the trees turn to charcoal. (yes)) When making hot chocolate clear water turns brown when you mix cocoa in (no) An old wheelbarrow is left out in the rain and rusts (yes) Water forming on the outside of a cold glass of water (no) Ice cream melting (no) A raw egg gets cooked (yes) When Alka Sletzer is dropped into water it fizzes (yes) Soda fizzes and bubbles rise as you pour it in a glass (no) When you crack a glow stick, hydrogen peroxide mixes with other chemicals causing it to glow and warm up (yes) Parts of a Reaction Equation Chemical equations show the conversion of reactants (the molecules shown on the left of the arrow) into products (the molecules shown on the right of the arrow). A + sign separates molecules on the same side The arrow is read as “yields” Example C + O2 CO2 This reads “carbon plus oxygen react to yield carbon dioxide” The charcoal used in a grill is basically carbon. The carbon reacts with oxygen to yield carbon dioxide. The chemical equation for this reaction, C + O2 CO2, contains the same information as the English sentence but has quantitative meaning as well. Chemical Equations Because of the principle of the conservation of matter, an equation must be balanced. It must have the same number of atoms of the same kind on both sides. Lavoisier, 1788 Symbols Used in Equations Solid (s) Liquid (l) Gas (g) Aqueous solution (aq) H2SO4 Catalyst Escaping gas () Change of temperature () Balancing Equations When balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent) The SEVEN Diatomic Molecules Hydrogen (H2) Nitrogen (N2) Oxygen (O2) Fluorine (F2) Chlorine (Cl2) Iodine (I2) Bromine (Br2) Subscripts vs. Coefficients The subscripts tell you how many atoms of a particular element are in a compound. The coefficient tells you about the quantity, or number, of molecules of the compound. Chemical Equations 4 Al(s) + 3 O2(g) ---> 2Al2O3(s) This equation means 4 Al atoms + 3 O2 molecules ---produces---> 2 molecules of Al2O3 AND/OR 4 moles of Al + 3 moles of O2 ---produces---> 2 moles of Al2O3 Steps to Balancing Equations There are four basic steps to balancing a chemical equation. 1. Write the correct formula for the reactants and the products. DO NOT TRY TO BALANCE IT YET! You must write the correct formulas first. And most importantly, once you write them correctly DO NOT CHANGE THE FORMULAS! 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. 4. Check your answer to see if: The numbers of atoms on both sides of the equation are now balanced. The coefficients are in the lowest possible whole number ratios. (reduced) Some Suggestions to Help You Some Helpful Hints for balancing equations: 1. Take one element at a time, working left to right except for H and O. Save H for next to last, and O until last. 2. IF everything balances except for O, and there is no way to balance O with a whole number, double all the coefficients and try again. (Because O is diatomic as an element) 3. (Shortcut) Polyatomic ions that appear on both sides of the equation should be balanced as independent units Balancing Equations 2 H2(g) + ___ O2(g) ---> ___ 2 H2O(l) ___ What Happened to the Other Oxygen Atom????? This equation is not balanced! Two hydrogen atoms from a hydrogen molecule (H2) combines with one of the oxygen atoms from an oxygen molecule (O2) to form H2O. Then, the remaining oxygen atom combines with two more hydrogen atoms (from another H2 molecule) to make a second H2O molecule. Balancing Equations 2 Al(s) + ___ 3 Br2(l) ---> ___ Al2Br6(s) ___ Balancing Equations ____C3H8(g) + _____ O2(g) ----> _____CO2(g) + _____ H2O(g) ____B4H10(g) + _____ O2(g) ----> ___ B2O3(g) + _____ H2O(g) Balancing Equations Sodium phosphate + iron (III) oxide sodium oxide + iron (III) phosphate Na3PO4 + Na2O + Fe2O3 ----> FePO4