answer
advertisement

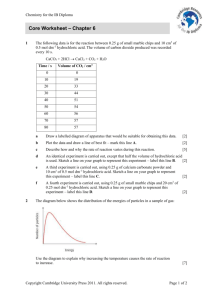
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 1. What always happens in a chemical reaction? answer A new substance is always formed in a chemical reaction 2. What changes might indicate that a chemical reaction has taken place? answer Energy change Permanent colour change Gas produced (effervescence) Solid formed in a liquid (precipitation) 3. An element is ……… answer An element is the simplest type of substance. It is made up of only 1 type of atom. 4. What is a GROUP in the Periodic Table? answer A column of elements 5. What is a PERIOD in the periodic table? answer A row of elements 6. Why are certain elements in the same group? answer Because they have similar chemical properties 7. Give some metallic properties? answer Conduct electricity and heat Malleable (bend into shape) Ductile (draw into wires) Most of them are strong 8. What are compounds? answer Substances made of two or more different types of atom joined together. 9. What is the difference between a compound and a mixture? answer The atoms of a compound are joined together. The substances in a mixture are NOT joined and can be easily separated. 10. What is a molecule? answer Two or more non metal atoms joined together Can be element e.g. O2, H2 OR compound e.g. H2O, CO 11. What does -ide at the end of the name of a compound indicate? answer IDE means that the compound is made up of the TWO elements (the actual elements are obvious from the compound’s name). 12. What is ELECTROLYSIS? answer Using ELECTRICITY to break up a compund. 13. Write a word equation for: Methane burns in oxygen to produce carbon dioxide. Water is also produced. answer Methane + Oxygen Carbon + Water Dioxide 14. Sort these into chemical answer reaction and physical change. Hair growing, Chemical Reaction Ice melting, Match burning, Hair growing Baking cake, Firework exploding,Match burning Boiling water Baking cake Firework exploding Physical Change Ice melting Boiling Water 15. Describe how the concentration of reactants affects the speed of a reaction? As reactant concentration increases the speed of reaction increases. 16. What is meant by a property of a chemical? answer A property of a chemical is what it is like or what it can do chemically 17. Name FOUR factors which affect the speed (or rate) of a chemical reaction? Particle Size (Surface Area) Temperature Concentration Use of Catalyst 18. When can a catalyst NOT speed up a reaction? SOME reactions DO NOT have a catalyst. 19. Where in the Periodic Table would you find the METALS and the NON-METALS? answer Metals are found to the Left Hand Side of the zigzag line; Non-metals to the Right Hand Side of the zig-zag line 20. What are ATOMS? answer Atoms are very small, particles which make up every element. They are too small to see. 21. What is a PRECIPITATE? answer A precipitate is a solid which is formed when two solutions react together 22. What is EFFERVESCENCE? answer Effervescence is bubbling and fizzing when a gas is given off . 23. Use the Periodic Table in your planner. Give the SYMBOLS for the elements : lead, gold, sodium, potassium and iron? Lead – Pb Gold – Au Sodium – Na Potassium – K Iron - Fe answer 24. Describe how particle size affects the speed of reaction? As particle size decreases the speed of reaction increases 25. Describe how temperature affects the speed of reaction? As temperature increases the speed of reaction increases 26. What are ENZYMES? Catalysts from living things (Biological catalysts) 27. What is a catalyst? A substance which can speed up a reaction, but is not used up in the reaction and can be recovered chemically unchanged 28. Why does decreasing the particle size of reactants increase the speed of reaction? Smaller particles give a bigger surface area. This results in more collisions between reactants and therefore faster reactions 29. What must be controlled when carrying out experiments to compare speeds of reaction? All variables other than the one being tested must be kept the same in all experiments 30. What is a VARIABLE? Any factor in an experiment which can be changed or varied. e.g. concentration or temperature of the solution used 31. Rates Experiments • Which two of the following experiments could be used to find the effect of changing the particle size? A 2M hydrochloric acid at 250C B 2g of small chalk lumps answer C 2M hydrochloric acid at 250C 2g of large chalk lump 2M hydrochloric acid at 150C 2g of small chalk lumps D 1M hydrochloric acid at 250C 2g of small chalk lumps 32. Rates Experiments • Which two of the following experiments could be used to find the effect of changing the concentration of the acid? A 2M hydrochloric acid at 250C B 2g of small chalk lumps answer C 2M hydrochloric acid at 250C 2g of large chalk lump 2M hydrochloric acid at 150C 2g of small chalk lumps D 1M hydrochloric acid at 250C 2g of small chalk lumps 33. Rates Experiments • Which two of the following experiments could be used to find the effect of changing the temperature of the acid? A 2M hydrochloric acid at 250C B 2g of small chalk lumps C answer 2M hydrochloric acid at 250C 2g of large chalk lump 2M hydrochloric acid at 150C 2g of small chalk lumps D 1M hydrochloric acid at 250C 2g of small chalk lumps