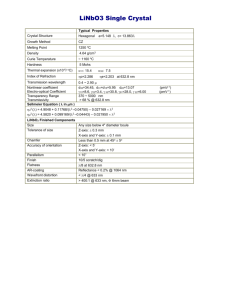
LiNbO3 workshop talk Sept 2013
advertisement

Computer modelling of structural and defect properties of stoichiometric lithium niobate Robert A Jackson School of Physical & Geographical Sciences Keele University Keele, Staffordshire ST5 5BG, UK r.a.jackson@keele.ac.uk http://www.robajackson.com 1 Acknowledgements Mário Valerio, Romel Araujo (Aracaju, Brazil) László Kovács, Krisztián Lengyel (Budapest, Hungary) Günter Borchardt, Peter Fielitz (Clausthal, Germany) Bud Bridges (Santa Cruz, USA) … and the workshop organisers for the invitation! International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 2 Relevant publications (some hard copies available) • [1] R A Jackson, M E G Valerio ‘A new interatomic potential for the ferroelectric and paraelectric phases of LiNbO3’ Journal of Physics: Condensed Matter, 17, 837-843 (2005) • [2] R M Araujo, K Lengyel, R A Jackson, L Kovács, M E G Valerio ‘A computational study of intrinsic and extrinsic defects in LiNbO3’ Journal of Physics: Condensed Matter, 19, 046211 (2007) • [3] R M Araujo, M E G Valerio, R A Jackson ‘Computer modelling of trivalent metal dopants in lithium niobate’ Journal of Physics: Condensed Matter, 20, 035201 (2008) International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 3 Plan of talk • • • • • Motivation & background Brief introduction to methodology Potential derivation & structural properties Defect properties Ongoing & future work International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 4 Motivation & background • The interatomic potential published by Donnerberg and coworkers in 1989-90 was widely used. However: (i) advances in computational software and (ii) the continued interest in the material and the availability of new experimental data prompted us to revisit and re-derive the potential (published in 2005). International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 5 Potential derivation • The potential was fitted to simultaneously reproduce the structures of LiNbO3a, Li2O and Nb2O5 to allow consistency in later defect calculations. • The GULP codeb was used, employing the free energy option (allowing temperature dependence of the structure to be treated). a S C Abrahams, P Marsh, Acta Cryst., B 42, 61 (1986) b J Gale, see: http://projects.ivec.org/gulp/ International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 6 Brief details of the potential* • Full ionic charges on Li, Nb and O. • Buckingham potentials describe the interactions between Li-O, Nb-O & O-O. • A shell model is employed for O. • A 3-body bond bending potential describes the ONb-O interactions. *R A Jackson, M E G Valerio, J Phys.: Condensed Matter, 17, 837 (2005) International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 7 Structural agreement Ferroelectric phase Exp. [1] This work Donnerberg potential 0K 295 K 0K 295 K a=b 5.1474 5.1559 5.1868 5.2271 5.2631 c 13.8561 13.6834 13.7103 14.2730 14.2167 Paraelectric phase Exp. [2] This work Donnerberg potential 0K 293 K 0K 293 K a=b 5.2924 5.1530 5.0919 2.3030 2.3042 c 13.8462 13.8418 13.2111 5.6412 5.6402 [1] S C Abrahams, P Marsh, Acta Cryst. B, 42, 61 (1986) [2] H Boysen, F Altorfer, Acta Cryst. B, 50, 405 (1994) International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 8 Lattice parameter as a function of temperature T/K aexp (Å) acalc (Å) a () cexp (Å) ccalc (Å) c () 0 - 5.1559 - - 13.6834 - 10 - 5.1745 - - 13.7035 - 295 5.1474 5.1868 0.77 13.8561 13.7103 -1.06 297 5.1483 5.1864 0.74 13.8631 13.7101 -1.10 523 5.1700 5.2133 0.84 13.8700 13.7200 -1.08 International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 9 Modelling defect properties • Using the Mott-Littleton method, energies of formation of the intrinsic defects in LiNbO3 were calculated. • These allow predictions to be made about the defect chemistry of the material. (See Araujo et al: Journal of Physics: Condensed Matter, 19, 046211 (2007)) International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 10 Mott-Littleton approximation © Mark Read Region I Ions are strongly perturbed by the defect and are relaxed explicitly with respect to their Cartesian coordinates. Region II Ions are weakly perturbed and therefore their displacements, with the associated energy of relaxation, can be approximated. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 11 Formation energies for basic defects (in stoichiometric LiNbO3) Defect 0K 293 K * [1] model II VLi’ 9.81 9.71 9.8 VNb’’’’’ 127.56 127.45 117.3 VO 18.98 18.91 19.5 Lii -7.08 -7.12 -8.87** Nbi -104.12 -104.25 -110.68** Oi’’ -9.47 -9.64 -16.08** NbLi -98.37 -98.49 -99.5 LiNb’’’’ -113.99 [1] Donnerberg et al, Phys. Rev. B., 40, 11909 (1989) * Temperature taken into account via lattice expansion. ** Deduced values since paper does not report these values. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 12 Frenkel, Schottky and pseudo-Schottky energies* (per defect) Defect 0K 293 K * [1] Li Frenkel 1.37 1.30 0.93 Nb Frenkel 11.72 11.60 6.26 O Frenkel 4.76 4.64 3.42 Schottky LiNbO3 3.95 3.85 3.91 PseudoSchottky Li2O 1.81 1.80 1.94 PseudoSchottky Nb2O5 5.09 5.07 2.85 International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 *Calculated for information only since defects are more complex. Expected trends in values are observed. 13 Models to explain the observed experimental data • The simple Frenkel and Schottky models do not explain the observed behaviour in LiNbO3. • For example, the NbLi + 4VLi’ defect cluster has a formation energy of –63.61 eV. • We needed to consider possible reactions that give rise to such defects. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 14 Explaining the observed nonstoichiometry • Following the work of Kovács and Polgár*, we considered models based on antisite or interstitial Nb compensated by Li or Nb vacancies. • 3 possible reactions were considered (see next slide): * L Kovács and K Polgár, Crystal Research and Technology, 21, K101 (1986) International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 15 Possible defect reactions that give rise to Li deficiency Antisite Nb compensated by Li vacancies 5LiLi + ½Nb2O5 4V’Li + NbLi + 5/2Li2O E(reaction) = -0.98 (-2.52*) eV per Li2O formula unit Antisite Nb compensated by Nb vacancies 5LiLi + 4NbNb + ½Nb2O5 5NbLi + 4VNb’’’’’ + 5/2Li2O E(reaction) = 29.8 eV per Li2O formula unit Interstitial Nb compensated by Li vacancies 5LiLi + ½Nb2O5 5VLi’ + Nbi + 5/2Li2O E(reaction) = 0.49 eV per Li2O formula unit * ‘Bound’ defect configuration International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 16 Conclusions from the reactions • If the reaction energies are calculated, using the basic defect energies already obtained, we concluded that: – only the antisite Nb/Li vacancy model is energetically favourable. – of the other two mechanisms, the interstitial Nb/Li vacancy model is more favourable than the antisite Nb/Nb vacancy model. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 17 Divalent and trivalent dopants • The incorporation of a range of dopant ions in LiNbO3 was modelled. • Divalent and trivalent ion substitution was considered. • Charge compensation is needed for substitution at either the Li+ or Nb5+ site. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 18 Dopant ions considered • Reference [2]: M2+ dopants Mg, Mn, Fe, Co, Ni, Zn, Sr, Cd, Ba & Pb, and M3+ lanthanide dopants Ce-Lu. • Reference [3] focused on M3+ dopants: Sc, Cr, Fe and In. [2] Journal of Physics: Condensed Matter, 19, 046211 (2007) [3] Journal of Physics: Condensed Matter, 20, 035201 (2008) International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 19 Summary of modelling procedure • The GULP code is used to calculate the substitution energies, e.g. M2+ at the Li+ site, denoted by MLi in Kroger-Vink notation. • The substitution energies are then converted into solution energies, which give the total energy involved in the process: International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 20 Solution energies • Assuming M2+ substitution at the Li+ site, a possible scheme could be: MO + 2 LiLi → MLi + VLi’ + Li2O • This assumes charge compensation by Li vacancies, but other possibilities are considered. • The same idea is applied to M3+ dopants. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 21 Predicted doping schemes: M2+ ions • From the calculations, the following predictions are made based on lowest energies: • Co-doping at both Li+ and Nb5+ sites, except for Fe2+ and Cd2+ for which substitution at the Nb5+ site with charge compensation by Nb - Li antisite substitution is preferred. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 22 Predicted doping schemes: M3+ ions • The predicted scheme for all the lanthanide ions and Sc, Cr and Fe is self-compensation: M2O3 + LiLi + NbNb → MLi + MNb’’ + LiNbO3 • For In, the preferred scheme involves doping at the Nb5+ site with charge compensation by Nb-Li anti-sites. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 23 Further calculations – M4+ & co-doping • Calculations have also been performed on Hf4+ doping, and a range of pairs of co-doped ions. • These results will be submitted for publication, possibly in the ‘Lithium Niobate: Properties and Applications’ special journal issue being planned. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 24 Some relevant experimental data • Studies of M2+ & M3+ dopants in LiNbO3 have included: Mn2+ - LiNbO3: Darwish et al, NIMB, 141, 679-683 (1998) Supports the idea of Mn2+ self compensation; does not give dopant concentration. Mg2+ - LiNbO3: González-Martínez et al, Opt. Comm., 282, 1212-1219 (2009) Dopant concentration 0.0714-0.2422 mol%; suggests that self compensation occurs ‘after a certain dopant concentration level’. Er3+, Cr3+ - LiNbO3: Dierolf & Sandmann, J. Lum., 125, 67-79 (2007) Mainly assumes Li site occupancy, but dopant concentration is unclear as several samples have been used. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 25 Comparison with recent experimental data • Bud Bridges recently published a paper: ‘EXAFS evidence for a primary ZnLi dopant in LiNbO3’ (F Bridges et al, Phys. Rev. B 85 064107 (2012)) • This doesn’t find any Zn at the Nb site, but may not be directly comparable with the calculations (concentration effects, stoichiometry of sample?) • EXAFS measurements on In have also been performed. International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 26 General comments on comparison with experimental data • The calculation results reported are at infinite dilution, so no concentration effects are considered. • In recent work we are looking at finite dopant concentrations in other materials, and this could be done for dopants in LiNbO3 (needs persons and €€€). • There may be issues with the stoichiometry of the older crystal samples (i.e. are we comparing ‘like with like’?) International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 27 Ongoing and future work • Results on tetravalent dopants and co-doping is still to be published. • Bud Bridges has detailed EXAFS data on Zn & In doped LiNbO3, which we are hoping to reproduce. • We are in discussions with Peter Fielitz and Günter Borchardt about modelling defects using some different reaction schemes (talk at 12:00 tomorrow). International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 28 Thank you! International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 29 International Workshop on Stoichiometric Lithium Niobate, Goslar, Germany, September 2013 30