Slide 1
advertisement
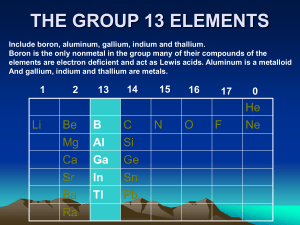
P- BLOCK ELEMENTS 1) The elements belonging to groups 13 to 18 constitute p block elements. 2)The general electronic configuration for the atoms of this block is ns²np¹. 3) The group 13 of the periodic table consists of elements boron , aluminium, gallium, indium and thallium. 4) Except boron, which is classified as a non metat, all other elements of this group are metals. GENERAL CHARACTERSTICS OF GROUP 13 ELEMENTS 1) ATOMIC AND IONIC RADII : On moving down the group, the atomic and ionic radii increases as no. of shells increases. 2) MELTING AND BOILING POINT : The melting and boiling point decreases on moving down the group. 3) IONISATION ENERGY : On moving down the group,ionisation energies decreases. 4) ELECTROPOSITIVE CHARACTER : The group 13 elements have less electropositive character. 5) TENDENCY TO FORM COVALENT COMPOUNDS :In group 13 elements boron shows covalent character, the other elements show ionic character. Comparative study of compounds of group 13 elements 1) HYDRIDES: Boron forms a no. of hydrides having the general formula BnHn+4 and BnHn+6. The compounds of boron and hydrogen are called boranes. These contain special types of bonds known as multicentre bonds. 3NaBH4 + AlCl3 ------------ Al[(BH4)3] + 3NaCl 2) OXIDES AND HYDROXIDES: All the elements of group 13 form oxides of general formula M2O3. For e.g. B2O3 , AL2O3. The oxide of boron is B2O3 and known as boric oxide. 2H3BO3 ------------ B2O3 +3H2O The oxides and hydroxides of boron are weakly acidic and therefore , react with alkalies B2O3+ NaOH--------- 2NaBO2 + H2O Sodium metaborate 3)OXO ACIDS :- Boric acids Among the oxyacids of group 13 elements, orthoboric acids B(OH)3 or H3BO3 is important. Some boric acids are known for e.g. 1) Orthoboric acid H3BO3 2)Metaboric acid HBO2 3)pyroboric acid H₆B₄O₉ 4)Tetraboric acid H₄B₄O₇ Structure of Boric acid: 1) The electronic configuration of boron atom is 2s² 2p¹. 2) In orthoboric acid, BO₃³- units are bonded together through hydrogen bonds into twodimensional sheet. 3) The hydrogen bonding in one H₃BO₃ unit is shown in fig (1) and complete structure is shown in fig (2). 4) It is evident that each boron atom remains bonded to three oxygen atoms and each oxygen atom is bonded to a hydrogen atom. CHEMISTRY OF SOME IMPORTANT COMPOUNDS DIBORANE: It is the simplest of the boranes and it forms the starting material for the preparation of other boranes. PREPARATION OF DIBORANE 1)It can be prepared in small quantities by the reaction of iodine on sodium borohydride in diglyme [( CH2OCH2CH2)2O]. 2NaBH4 + I2----------> B2H6 + 2NaI + H2 2) It can also be prepared by the reduction of boron halides with reducing agents like LiH, NaH, CaH2etc.in ether solution. 8BF3 + 6LiH ----------> B2H6 + 6LiBF4 2BCl3 + 6NaH---------> B2H6 + 6NaCl 3) It can be prepared by passing silent electric discharge through a mixture of hydrogen and boron trichloride at low pressure. 2BCl3 + 6H2O----------- > B2H6 + 6HCl 4) On an industrial scale, diborane is prepared by reducing gaseous BF3 with sodium hydride at 180⁰ C. 2BF3 + 6NaH-------------- > B2H6 + 6NaF PROPERTIES OF DIBORANE PHYSICAL STATE :- It is a colourless gas with a foul smell. 2) STABILITY : - It is stable only at low temperature when heated at temperatures b/w 100 ⁰ C and 250 ⁰ C, it changes to a no. of higher boranes. B2H6 ---------------- > B4H10, B5H9, B5H11, B6H10 etc. 3) ACTION OF OXYGEN :- It is spontaneously flammable and burns in oxygen liberating a lot of energy. B2H6 + 3O2---------- > B2O3 + 3H2O 4) HYDROLYSIS : - it gets readily hydrolysed yielding boric acid and hydrogen.so, it acts as a reducing agent. B2H6 + 6 H2O ------> 2H3BO3 + 6H2 5) ACTION WITH HALOGEN:B2H6 + Cl2----------> B2H5Cl + HCl B2H6 + HI---------- > B2H5I + H2 6) ACTION WITH HYDRIDES : 2NaH + B2H6--------> 2Na[BH4] 6) REACTION WITH PYRIDINE:- Diborane combines with pyridine to form salt. B2H6 + 2 C5H5N----------- > 2H3B⁻‹—N⁺C5H5 7) ACTION WITH BORON HALIDES:- Diborane reacts with boron halides to form halodiboranes B2H6 + BCl3---------------- > B2H5Cl + BHCl2 8) ADDITION TO ALKENES- HYDROBORATION REACTION :Diborane is added to the alkenes or alkynes in ether solvents at room temperature to form alkyl borane. 6RCH=CH2 + B2H6 --------- > 2(RCH-CH2)3B C2H5COOH (RCH-CH2)3B --------------- > 3RCH2 CH3 (alkane) H2O2/OH⁻ (RCH-CH2)3B ---------------- > 3RCH2CH2OH + H3BO3 (1⁰ alcohol) (boric acid) STRUCTURE AND BONDING OF DIBORANE From the electron diffraction and infra red spectroscopic studies, it has been concluded that diborane has the following structure— In diborane structure, 1) There are two types of hydrogen atoms. 2) Four hydrogen atoms, two on the left and two on the right known as terminal hydrogens, are different than the other two hydrogen atoms, known as bridging atoms. 3) The two boron atoms and the four terminal hydrogen atoms lie in the same plane. 4) While the two bridging atoms, one above and other below are in a plane at right angle to the rest of the molecule. NATURE OF BONDS IN DIBORANEThere are three centre electron pair bonds , B-H-B involving one electron pair bonds which binds three atoms : B, H and B.such a bond may be indicated as H…..B…..H. It was postulated that boron atoms undergoes sp3 hybridisation involving one 2s and all the three p orbitals including one empty orbital. The four sp3 hybrid orbitals adopt tetrahedral arrangements. The hybrid orbital containing an unpaired electron of one boron and the vacant hybrid orbital of the second boron atoms overlap simuntaneously with 1s orbital of a hydrogen atom to form a B…..H…..B bridge bond. It is called a three centre electron pair bond. This type of bond is also known as BANANA BOND. BORAZINE:It is isoelectronic with benzene because of its similarity in physical properties and structure, it has been known as inorganic benzene. 1)PREPARATION:it was first prepared by alfred stock in 1926 by the reaction of diborane and ammonia in the molar ratio of 1:2 at 250-300⁰C. 3B2H₆+ 6NH₃---------- 2B₃N₃H₆ +12H₂ 2) It can also be prepared by the direct reaction of alkali metal borohydrides with ammonium chloride. 3NaBH4+ 3NH4Cl------------- B₃N₃H₆+ 3NaCl + 9H₂ HALIDES OF AlCl₃:1) AlF3 IS PREPARED BY TREATING AI2O3 WITH HF GAS AT 700⁰ C. Al₂O₃ + 6HF------- 2AlF3+ 3H2O 2) 2Al + 3Cl2----2AlCl3 3) Al2O3+ 3Cl2+3C----- 2AlCl3+ 3CO 4) STRUCTURE OF AlCl3:5) In crystalline state, anhydrous AICI3 has closely packed str. 6) It exists as dimer in which there is four co- ordination around each Al atom. 7) Al is tetrahedrally surounded by four CI atoms. 8) In addition to three covalent bonds, two co-ordinate bonds are formed 9) By the donation of electron pair of CI to the vacant orbital of AI. 10) The dimer str. Exists in vapour state at low temp. but at higher temperature,it dissociates to trigonal planar in which co- ordination no. of AI is three. COMPARATIVE STUDY OF GROUP 16 ELEMENTS:1)HYDRIDES:- All elements of group 16 form halides such as H₂O, H₂S, H₂Se, H₂Te etc. The hydride of oxygen i.e. H₂O is available in abundance in nature. The hydrides of other elements can be obtained by the action of acids on metal sulphides, selenides and tellurides. FeS + H2SO4---------- FeSO4 + H2S k₂Se + H₂SO₄ ---------- K₂SO₄ + H₂Se 2) TRENDS IN CHARACTERSTICS OF HYDRIDES:1) Volatility :- All hydrides are volatile. The volatility increases from H₂O to H₂Se and then decreases. 2) Acidic character:- The hydrides of this group are weakly acidic.for e.g. H₂S H⁺+HS¯ HS¯ H⁺+ S 3) Thermal stability:- It decreases from H₂O to H₂Te as:H₂O > H₂S> H₂Se> H₂Te 4) Reducing character:- All hydrides except water are reducing agent. The reducing character increases from H₂O < H₂S < H₂Se < H₂Te OXYACIDS OF SULPHUR:1) Sulphurous acid, H₂SO₃ It contains sulphur in +4 oxidation state. It is a strong acid. It is diatropic and ionise in two stages. H₂SO₃ ------- H⁺ + HSO₃¯ HSO₃⁻ ----- H⁺+ SO₃²¯ STRUCTURE OF SULPHUROUS ACID :Sulphurous acid forms two types of isomeric diethyl derivative. They are symmetrical and unsymmetrical derivative. The sulphite ion has a pyramidal str. Involving sp³ hybridisation of S- atom in which one of the sp³ hybrid orbital occupies alone pair of electrons. Two sp³ hybrid orbital overlap with 2p-orbitals of oxygen forming two S-O bonds. The half filled d- orbital sulphur forms pπ- dπ bond with third O- atom. 2) SULPHURIC ACID:It contains +6 oxidation state. Because of its wide applications in industry, it is called as king of chemicals. H₂SO₄ H₂O + SO₃ ACIDIC CHARACTER:H₂SO₄ H₂SO₄ H⁺ + HSO₄ 2H⁺ +SO₄²¯ STRUCTURE:It is dibasic acid.it has two hydrogens linked to oxygen atoms forming hydroxy groups. 1) PCI₅ THIOSULPHURIC ACID:It contains S in +2 oxidation state. H₂S₂O₃ -------- H₂SO₃ + S STRUCTURE:It is derived from sulphuric acid by the replacement of one of the oxygen atoms by a sulphur atom. DISULPHURIC ACID:It is a stronger oxidising agent and more powerful dehydrating agent. It combines with water to give sulphuric acid. H₂S₂O₇ + H₂O ------- 2H₂SO₄ PEROXODISULPHURIC ACID, H₂S₂O₈:It contains S in +6 oxidation state. It is also called MARSHALL’S ACID. STRUCTURE:- HYDROGEN PEROXIDE H₂O₂:It was discovered by french chemist J. L. THENARD in l8l8. Physical properties:1) It is a thick syrupy liquid with pale blue colour. 2) It is more viscous, less volatile and dense than water. 3) Its density is 1.44 g cm⁻³ 4) Its melting point is 272.4 K and boiling point is 358 k at 68 mm of Hg pressure. CHEMICAL PROPERTIES:1) DECOMPOSITION:- 2)ACIDIC NATURE:-It is a weak acid and dissociates as:- USES OF HYDROGEN PEROXIDE:1) It is used in industry as a bleaching agent for textiles, paper, pulp, straw leather, oils fat etc. 2) It is used as ahair bleach. 3) It is used as a antiseptic for washing wounds, teeth and ears under the name PERHYDROL. 4) It is used for restoring the colour of lead paintings. 5) It is used for preserving milk, wine and other liquors. 6) Recently it is used in environmental chemistry such as in pollution control treatment of domestic and industrial effluents ; oxidation of cyanides and restoration of aerobic conditions of sewage wastes. STRUCTURE OF HYDROGEN PEROXIDE:Hydrogen peroxide has a skew structure in which 2 hydrogen atoms are arranged in two directions almost perpendicular to each other and to the axis joining the two oxygen atoms. The fact that two O—H bonds lie in different planes is due to repulsion b/w the various bonding and non bonding orbitals. There is free rotation about O—O bond because there is no barrier to internal rotation.