Slide 1
advertisement

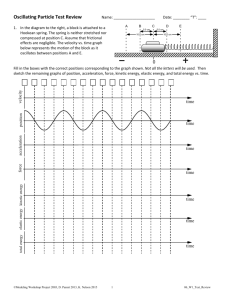
Name ____________________________________________ Date _________________ Period ________ OSCILLATING REACTIONS The world of science is, as you might have already noticed, an odd one. There are rules upon rules upon rules, but we seem to find that for every rule there is an exception. For example, all mammals give live birth, excepting, of course, the egg-laying mammals called monotremes. There are only four extant (the opposite of extinct) monotremes: the duck-billed platypus, and three types of spiny anteaters. All belong to the taxonomic order Monotremata. As an irrelevant yet didactic item, you may be interested to know that a baby monotreme is called a puggle. “This is all good,” you might say, “but what has it got to do with chemical reactions?” It serves to demonstrate the principle that when there is an exception to Nature’s rules, it is usually quite spectacular. Oscillating reactions are just that; spectacular, and, in their own way, a bit of an exception to many of Nature’s rules. In a “standard” chemical reaction, two or more reactants are combined, and they form a product. The reason for this often has to do with energy. In order to demonstrate this, follow the brief yet sprightly procedure below: I. Two students should stand on their chars. Do this carefully, as you will gain potential energy by elevating yourself. Do not prematurely turn this into kinetic energy! II. In this state, the two students are “less stable,” because they have greater potential energy. That is, they are more likely to fall than if they were sitting on their chairs. II. The two students should carefully sit down. Now, they are in a more stable state. The students represent the reactants. They started off in a state with more energy, and after the “reaction” (the act of sitting down), they had less energy, and represented the products. As it stands (or sits, as the case may be…), the products are not going to spontaneously turn back into the reactants, because it would take energy to do so, just as it would take energy for the students from the demonstration above to stand up again. We can represent one of these reactions as follows: A+BC Which would be read “A plus B yields C,” and where A and B are reactants, while C is a product. There are, of course, reactions that take in energy as well. Such reactions take place commerciallyavailable ice packs that become cold when two chemicals inside them are combined. Still, the products are not likely to just turn back into the reactants, as more energy would need to be used. The salient point here is that things in nature tend to want to be in the most stable form possible, and will not simply bounce back into a less stable form at the drop of a hat (or any other bit of haberdashery). This, then, is where oscillating reactions differ. You see, when they are completed, they don’t just stay, there, they “bounce back,” forming the reactants again. These oscillations can continue on for many minutes in some cases. The basic idea in many of these reactions is that they are actually two processes going on at the same time. The products of one process, however, become the reactants of the other! This can be represented thusly: A+BC D+EF F + C A + B, D + E One might choose to think about the concept in this manner: Suppose that you are a mean-spirited person, and you have a doll. You also have a friend (who is a lot nicer than you) who only likes dolls with no hair. You, being mean spirited, decide to shave your doll’s head. This triggers your friend to want the doll, and it is taken from you. You are mean-spirited, however, so you steal it back. Your friend still likes bald dolls, so the item is again purloined. And so the doll goes, back and forth, just like an oscillating reaction. In this manner, the two processes, viz. the stealing of the doll and the taking back, will continue until one or both parties becomes exhausted. The same thing happens in oscillating reactions. The conditions that favour one process will prevail (like you stealing the doll), and then the reaction will shift the conditions to favour the other process (like the doll being stolen). One of the most exciting of these oscillating reactions is the Briggs-Rauscher reaction. In this reaction, a color change is observed as the processes in the reaction go back and forth. The blue colour is formed when iodine is formed and reacts with starch in the solution. Another process uses up iodine molecules and thus the solution turns first amber, and then clear. The conditions then again favour the production of iodine, and the process repeats. Another slice of interesting trivia is that the conductivity of the solution changes as the reaction either consumes or produces various molecules. Conductivity is a measure of how well electricity will travel through a substance. A copper wire, for example, has a high conductivity. A brick has a very low conductivity. Indeed, were you to graph the conductivity of the reaction as it proceeded, the graph would take the shape of a regular wave; going up and down at even intervals. The actual stoichiometry (measurement of the relationships of the reactants and products) of this reaction is quite complex, so the above paragraph is what one might call an “executive summary.” The real explanation would require the wholesale slaughter of many trees just to get enough paper to write it out. We are going to do this reaction, but it behooves you to know a little bit about it and other reactions of this type before we continue. So, please read the directions below, and complete this assignment by the end of the class. Your task is to write what is known as a “précis” about oscillating reactions, with a focus on the Briggs-Rauscher reaction. A précis (a word derived from the French for “precise”) is a short summary of a topic. It is, as its name implies, not long, and yours should be no longer than one page. DO NOT make a list of these items; rather, construct a micro-essay, using several short-ish paragraphs. Make sure that you include the following items: 1. What makes an oscillating reaction different from a standard, one-way reaction. 2. How oscillating reactions are started and stopped. 3. Give at least three examples (other than the Briggs-Rauscher reaction) of oscillating reactions. 4. Explain how oscillating reactions can be used to model biological populations. That is, how does this constant back-and-forth action relate to groups of predators and prey? 5. Briefly explain how the Briggs-Rauscher reaction works IN YOUR OWN WORDS. 6. See if you can find any exciting videos or pictures of these reactions in action! Your response will be graded on its accuracy, precision, and the Conventions of Standard Written English. That means dot your i’s, cross your t’s, and make sure that none of your participles dangle. You may use the computers in the classroom and in the pod for research. I understand that much of what you might find on the interweb may be quite advanced, but I have faith that you can glean what you need to out of it. Please note that you do not need to cite your sources, but I expect, as always, that the work you hand in will be your own. That means you SHOULD NOT cut and paste anything. If you are working as a group, make sure that your finished product reflects the effort of all group members, as all group members will receive identical grades. Good luck, have fun, and learn much!