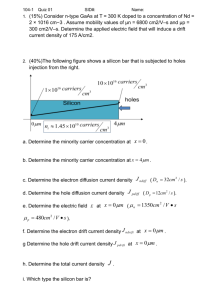
File
advertisement

Introduction • Electronics is defined as the science of the motion of charges in a gas, vacuum, or semiconductor. • Today, electronics generally involves transistors and transistor circuits • Microelectronics refers to integrated circuit (IC) technology, which can produce a circuit with multimillions of components on a single piece of semiconductor material. History of Semiconductors 1821: Thomas Seebeck discovered semiconductor properties of PbS 1833: Michael Faraday reported on conductivity temperature dependence of semiconductors 1875: Werner von Siemens invented a selenium photometer 1878: Alexander Graham Bell used this device for wireless optical communications 1907 Round demonstrated the first LED (using SiC) 1940 Russell Ohl discovered a p-n junction diode History of Semiconductors • Russell Ohl – Inventor of a p-n junction (1940) • In 1939, vacuum tubes were state of the art in radio equipment. Most scientists agreed tubes were the future for radio and telephones everywhere. • Russell Ohl didn't agree. He kept right on studying crystals, occasionally having to fight Bell Labs administration to let him do it. History of Semiconductors 1947: Bardeen, Brattain, and Shockley discovered a Bipolar Junction transistor First Integrated Circuit is Invented by Jack Kilby : 1958 First Transistor, 1947 The Nobel Prize in Physics 1956 Intel’s 1.7 Billion Transistor Chip 2004 Brief History • In December 1947, the first transistor was demonstrated at Bell Telephone Laboratories by William Shockley, John Bardeen and Walter Brattain. • From then until 1959, the transistor was available only as a discrete device, so the fabrication of circuits required that the transistor terminals be soldered directly to the terminals of other components. • In September 1958, Jack Kilby of Texas Instruments demonstrated the first integrated circuit fabricated in germanium. At about the same time, Robert Noyce of Fairchild Semiconductor introduced the integrated circuit in silicon. Brief History • 1954, Chapin, Fuller, and Pearson developed a solar cell. • 1958, John Kilby, invented the Integrated Circuit (IC). • 1958, Leo Esaki discovered a tunnel diode (Esaki diode). • 1960, Kahng and Atalla demonstrated the first MOSFET. • 1962, three groups headed by Hall, Nathan, and Quist demonstrated a semiconductor laser. • 1963, Gunn discovered microwave oscillations in GaAs and InP (Ridley-Watkins-Hilsum-Gunn effect). • 1963, Wanlass and Sah introduced CMOS technology Brief History • The development of the IC continued at a rapid rate through the 1960s, using primarily bipolar transistor technology. • Since then, the metal-oxide-semiconductor field-effect transistor (MOSFET) and MOS integrated circuit technology have emerged as a dominant force, especially in digital integrated circuits. • Device size continues to shrink and the number of devices fabricated on a single chip continues to increase at a rapid rate. • Today, an IC can contain arithmetic, logic, and memory functions on a single semiconductor chip. • The primary example of this type of integrated circuit is the microprocessor. Passive and Active Devices • In a passive electrical device, the time average power delivered to the device over an infinite time period is always greater than or equal to zero. • Resistors, capacitors, and inductors, are examples of passive devices. • Active devices, such as dc power supplies, batteries, and ac signal generators, are capable of supplying particular types of power. • Transistors are also considered to be active devices in that they are capable of supplying more signal power to a load than they receive. Electronic Circuits Schematic of an electronic circuit with two input signals: the dc power supply input, and the signal input Atomic Structure An atom is composed of : • Nucleus (which contains positively charged protons and neutral neutrons) • Electrons (which are negatively charged and that orbit the nucleus) Valence Electrons • Electrons are distributed in various shells at different distances from nucleus • Electron energy increases as shell radius increases. • Electrons in the outermost shell are called valence electrons • Elements in the period table are grouped according to the number of valence electrons • The valence electrons are shared between atoms, forming what are called covalent bonds At room temperature, some of the covalent bonds are broken by thermal ionization. Each broken bond gives rise to a free electron and a hole, both of which become available for current conduction. Valence Electrons a portion of the periodic table Semiconductor Materials Elemental Semiconductors 1. Si Silicon 2. Ge Germanium Compound Semiconductors 1. GaAs Gallium arsenide 2. GaP Gallium phosphide 3. AlP Aluminum phosphide 4. AlAs Aluminum arsenide 5. InP Indium phosphide Elemental/Compound Semiconductors • Silicon (Si) and Germanium (Ge) are in group IV, and are elemental semiconductors • Galium arsenide (GaAs) is a group III-V compound semiconductors Silicon Crystal • At 0°K, each electron is in its lowest possible energy state, and each covalent bounding position is filled. • If a small electric field is applied, the electrons will not move silicon is an insulator Silicon Atom Diagram at 0°K Two-dimensional representation of single crystal silicon At T = 0 K; all valence electrons are bound to the silicon atoms by covalent bonding Silicon Atom Diagram at Ambiant Temp The breaking of covalent bond for T>0°K creating an electron in the conduction band and a positively charged “empty state” Intrinsic Silicon • If the temperature increases, the valence electrons will gain some thermal energy, and breaks free from the covalent bond • It leaves a positively charged hole • In order to break from the covalent bond, a valence electron must gain a minimun energy Eg: Bandgap energy Energy band • The energy Eν is the maximum energy of the valence energy band. • The energy Ec is the minimum energy of the conduction energy band. • The bandgap energy Eg is the difference between Ec and Eν • The region between these two energies is called the forbidden bandgap. • Electrons cannot exist within the forbidden bandgap. Energy band diagram a) Vertical scale is electron energy & horizontal scale is distance through the semiconductor, although these scales are normally not explicitly shown. b)The generation process of creating an electron in the conduction band and the positively charged “empty state” in the valence band Energy band diagram a) Vertical scale is electron energy & horizontal scale is distance through the semiconductor, although these scales are normally not explicitly shown. b)The generation process of creating an electron in the conduction band and the positively charged “empty state” in the valence band Energy gap The energy gap decreases with the increase in temperature and is given by EG( T) = EG0-T where = a constant, (depends on material nature) = 3.60 X 10-4 for silicon ; = 2.23 X 10-4 for germanium ; For germanium EG0 = 0.785eV; at 0°K For silicon EG0 = 1.21eV; at 0°K For germanium, EG(T) = 0.785 - 2.23 X 10-4T; & At room temperature (300°K), EG = 0.72 ev. For silicon, EG(T) = 1.21 - 3.60 X 10-4T; & At room temperature (300°K), EG= 1.1 ev. Energy-band structure of (a) an insulator, (b) a semiconductor, and (c) a metal. Insulators/Conductors • Materials that have large bandgap energies (in the range of 3 to 6 electron-volts (eV)) are insulators, because at room temperature, essentially no free electron exists in the material • Materials that contain very large number of free electrons at room temperature are conductors • In a metal, the conduction band is partially filled. These electron can move easily in the material and conduct heat and electricity (Conductors). Semiconductors • Most electronic devices are fabricated by using semiconductor materials along with conductors and insulators. • Silicon is by far the most common semiconductor material used for semiconductor devices and integrated circuits. • Other semiconductor materials are used for specialized applications. Semiconductors • In a semi-conductor at 0 k the conduction band is empty and valance band is full. The band-gap is small enough that at room temperature some electrons move to the conduction band and material conduct electricity. • In a semiconductor, the bandgap energy is in the order of 1 eV. The net flow of free electrons causes a current. • In a semiconductor, two types of charged particles contribute to the current: the negatively charged electrons and the positively charged holes An electron–volt is the energy of an electron that has been accelerated through a potential difference of 1 volt, and 1 eV = 1.6 × 10−19 joules. Semiconductor In semiconductors, two types of charged particles contribute to the current: • the negatively charged free electron • the positively charged hole. • Two charge carrying particles (free electrons and holes) are formed along with a new electron-hole pair. • µn = mobility of the free electron (-ve charge carrying particle) • µp = mobility of the hole (+ve charge carrying particle) • Carriers A free electron is negative charge and a hole is positive charge. Both of them can move in the crystal structure. They can conduct electric circuit. • Recombination Some free electrons filling the holes results in the disappearance of free electrons and holes. • Thermal equilibrium At a certain temperature, the recombination rate is equal to the ionization rate. So the concentration of the carriers is able to be calculated. • In a pure semiconductor: no. of holes = no. of electrons i.e. n=p • The concentration of electrons and holes directly influence the magnitde of the current • In an intrinsic semiconductor (a single crystal semiconductor) the densities of holes and electrons are equal. Carrier concentration for n type Thermal equilibrium equation nn 0 pn 0 ni 2 Electric neutral equation nn 0 pn 0 N D Carrier concentration for p type Thermal equilibrium equation p p 0 n p 0 ni 2 Electric neutral equation p p0 n p0 N A Because the majority is much great than the minority, we can get the approximate equations shown below: nno N D 2 ni for n type pn 0 ND p p0 N A 2 ni for p type n p 0 NA • Carrier concentration in thermal equilibrium n p ni 3 EG kT ni BT e 2 • At room temperature(T=300K) ni 1.5 1010 carriers/cm3 B: constant related to specific semiconductor material Eg: Bandgap energy (eV) T: Temperature (°K) K: Boltzman Constant in eV/°K Semiconductor Constants • ni has a strong function of temperature. • The high the temperature is, the dramatically great the carrier concentration is. • At room temperature only one of every billion atoms is ionized. • Silicon’s conductivity is between that of conductors and insulators. • Actually the characteristic of intrinsic silicon approaches to insulators. • Electron density in the conduction band. • NC= 2.86 X 1019cm-3 for silicon and 4.7 X 1017cm-3 for gallium arsenide. • NV= 2.66 X 1019cm-3 for silicon and 7 X 1018cm-3 for gallium arsenide Fermi function Where n is in cm-3and N(E) is density of states in (cm3-eV) N(E) = γ(E – Ec)1/2 The probability that an electron occupies and electronic state with energy E is given by Fermi-Dirac distribution. Fermi function f(E) is : f(E) = 1/(1+e(E-Ef)/kT) Fermi distribution function F(E) versus (E–EF) for various temperatures. Mass Action Law n = p : number of electrons in CB = number of holes in VB This is due to the fact that when an electron makes a transition to the Conduction Band, it leaves a hole behind in Valance Band, having a bipolar (two carrier) conduction and the number of holes and electrons are equal. n.p = ni2 This equation is called as mass-action law. Intrinsic semiconductor. (a) Schematic band diagram. (b) Density of states. (c) Fermi distribution function. (d) Carrier concentration. n-Type semiconductor. (a) Schematic band diagram. (b) Density of states. (c) Fermi distribution function (d) Carrier concentration. Note that np= ni2. Extrinsic Semiconductor / Doping • Doped semiconductors are materials in which carriers of one kind predominate. • Only two types of doped semiconductors are available. • Conductivity of doped semiconductor is much greater than the one of intrinsic semiconductor. • The pn junction is formed by doped semiconductor. Extrinsic Semiconductor / Doping • The electron or hole concentration can be greatly increased by adding controlled amounts of certain impurities • For silicon, it is desirable to use impurities from the group III and V. • The phosphorus (group V) atom is called donor impurity because it donates an electron that is free to move • The boron (group III) has accepted a valence electron (or donated a hole), it is therefore called acceptor impurity • An N-type semiconductor can be created by adding phosphorus or arsenic N-Type Semiconductor Each dopant atom donates a free electron and is thus called a donor. The doped semiconductor becomes n type. A silicon crystal doped by a pentavalent element. A two-dimensional representation of the silicon crystal showing the movement of the positively charged “empty state” • In the figure, it appear as if a positive charge is moving through the semiconductor • This positively charged imaginary “particle” is called a hole. P-Type Semiconductor A silicon crystal doped with a trivalent impurity. Each dopant atom gives rise to a hole, and the semiconductor becomes p type. Conductivity of Semiconductor Total current density J with in the intrinsic semiconductor is given by J = Jn + Jp = qnµnE + qpµpE = (nµn + pµp)qE = σE σ is the conductivity of a semiconductor The resistivity (ρ) of a semiconductor is the reciprocal of conductivity, i.e., ρ = 1/σ. Conductivity of Semiconductor • For pure (intrinsic) semiconductor, n = p = ni (intrinsic carrier concentration). • Conductivity of an intrinsic semiconductor is σi = niq(µn + µp). Conductivity of N-and P-type semiconductors: • For N-type semiconductors, as n>>p, then the conductivity, σ = q n µn • For P-type semiconductors, as p>>n, then the conductivity, σ = q p µp Drift & Diffusion There are two mechanisms by which holes and free electrons move through a silicon crystal. Drift--- The carrier motion is generated by the electrical field across a piece of silicon. This motion will produce drift current. Diffusion--- The carrier motion is generated by the different concentration of carrier in a piece of silicon. The diffused motion, usually carriers diffuse from high concentration to low concentration, will give rise to diffusion current. Drift velocity and Current • Drift Drift velocities vp drift p E vn drift n E where q q n p n m , p m Drift current densities J n drift (qn) ( n E ) qn n E J p drift qp p E Where µn , µp are the constants called mobility of holes and electrons respectively. • Total drift current density J drift q(n n+p p ) E • Resistivity 1 q ( n + p ) n p • Resistivities for doped semiconductor 1 For n type qN D n 1 q(n p ) n p For p type 1 qN A p * Resistivities are inversely proportional to the concentration of doped impurities. • Temperature coefficient(TC) TC for resistivity of doped semiconductor is positive due to negative TC of mobility • Resistivity for intrinsic semiconductor 1 q (n p ) 1 qn ( ) n p i n p * Resistivity is inversely proportional to the carrier concentration of intrinsic semiconductor. • Temperature coefficient(TC) TC for resistivity of intrinsic semiconductor is negative due to positive TC of . • Diffusion A bar of intrinsic silicon (a) in which the hole concentration profile shown in (b) has been created along the x-axis by some unspecified mechanism. Cont… I diff n I diff p I diff dn qADnn qADn dx dp qAD p p qAD p dx I ndiff I pdiff qADnn D p p I T I diff I drift Diffusion currents only flow when there is a concentration difference for either the electrons or holes (or both). Einstein Relationship Einstein relationship exists between the carrier diffusivity and mobility: Dn Dp kT VT n p q Where VT is Thermal voltage. At room temperature, V T 25mv Diffusion length (L) • The average distance that on excess charge carrier can diffuse during its life time is called the diffusion length L L D • Where D is the diffusion coefficient that may be related to the drift mobility, µ, through the Einstein relation as D (kT / q) Diffusion length • Lp is the average distance a hole will move before recombining. • Ln is the average distance an electron will move before recombining. Ln Dn n Lp D p p Carrier life time • The carrier life time is defined as the time for which, on average, a charge carrier will exist before recombination with a carrier of opposite charge. • It depends on the temperature and impurity concentration in the semiconductor material. Carrier life time • The mean life times n , p of electrons and hole concentrations indicate the time required for the excessive electron and hole concentrations to return to their equilibrium values. dn n 0 n dt n dp p0 p dt p Continuity Equation Relating to the conservation of charge. Rate of hole build up = increase of hole concentration in the volume - the recombination rate The equation of conservation of charge, or the continuity equation, p p0 dp d p dp Dp 2 p dt p dx dx 2 Where Ɛ is the electric field intensity within the volume. • Considering holes in the n-type material, the subscript n is added to P and p0. • Also, since p is a function of both t and x, partial derivatives should be used. Making these changes, finally equation is, pn pn pno pn pn Dp p 2 t p x x 2