Trace Element Geochemistry: Definitions & Applications

Trace Elements - Definitions
• Elements that are not stoichiometric constituents in phases in the system of interest
– For example, IG/MET systems would have different “trace elements” than aqueous systems
• Do not affect chemical or physical properties of the system as a whole to any significant extent
• Elements that obey Henry’s Law ( i.e. has ideal solution behavior at very high dilution)
Graphical Representation of Elemental Abundance
In Bulk Silicate Earth (BSE)
Six elements make up 99.1% of BSE ->
The Big Six: O, Si, Al, Mg, Fe, and Ca
From W. M. White, 2001
Goldschmidt’s Geochemical Associations (1922)
• Siderophile: elements with an affinity for a liquid metallic phase (usually iron), e.g.
Earth’s core
• Chalcophile: elements with an affinity for a liquid sulphide phase; depleted in BSE and are also likely partitioned in the core
• Lithophile: elements with an affinity for silicate phases, concentrated in the Earth’s mantle and crust
• Atmophile: elements that are extremely volatile and concentrated in the Earth’s hydrosphere and atmosphere
Trace Element Associations
From W.M. White, 2001
Trace Element Geochemistry
• Electronic structure of lithophile elements is such that they can be modeled as approximately as hard spheres; bonding is primarily ionic
• Geochemical behavior of lithophile trace elements is governed by how easily they substitute for other ions in crystal lattices
• This substitution depends primarily by two factors:
– Ionic radius
– Ionic charge
Effect of Ionic Radius and Charge
Ionic Radii
• The greater the difference in charge or radius between the ion normally in the site and the ion being substituted, the more difficult the substitution.
• Lattice sites available are principally those of Mg, Fe, and Ca, all of which have charge of 2+.
• Some rare earths can substitute for Al 3+ .
Magnesium (Mg 2+ ): 65 pm
Calcium (Ca 2+ ): 99 pm
Strontium (Sr 2+ ): 118 pm
Rubidium (Rb + ): 152 pm
Values depend on Coordination Number
1 pm = 10 -12 m
1 Å = 10 -10 m
1 pm = 10 -2 Å
Classification of Based on Radii and Charge
Ionic Potential - charge/radius rough index for mobility
(solubility)in aqueous solutions:
<3 (low) & >12 (high) more mobility
1) Low Field Strength (LFS)
Large Ion Lithophile (LIL)
2) High Field Strength (HFS)
– REE’s
3) Platinum Group Elements
NB 1 Å = 10 -10 meters = 100 pm
More Definitions
• Elements whose charge or size differs significantly from that of available lattice sites in mantle minerals will tend to partition ( i.e.
preferentially enter) into the melt phase during melting.
– Such elements are termed incompatible
– Examples: K, Rb, Sr, Ba, rare earth elements (REE), Ta,
Hf, U, Pb
• Elements readily accommodated in lattice sites of mantle minerals remain in solid phases during melting.
– Such elements are termed compatible
– Examples: Ni, Cr, Co, Os
Trace element substitutions
The (Lanthanide) Rare Earth Elements
H He
Li Be
Na Mg
K Ca
Rb Sr
Cs
Fr
Ba
Ra
B C N O F Ne
Al Si P S Cl Ar
Sc Ti V Cr Mn Fe Co Ni Cu Zn
Y Zr Nb Mo Tc Ru Rh Pd Ag Cd
Ga Ge As Se Br Kr
In Sn Sb Te I Xe
La Hf
Ac
Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Rare Earth Element Behavior
• The lanthanide rare earths all have similar outer electron orbit configurations and an ionic charge of +3 (except Ce and Eu under certain conditions, which can be +4 and +2 respectively)
• Ionic radius shrinks steadily from La (the lightest rare earth) to Lu (the heaviest rare earth); filling f orbitals; called the “ Lanthanide Contraction ”
• As a consequence, geochemical behavior varies smoothly from highly incompatible (La) to slightly incompatible (Lu)
Rare Earth Element Ionic Radii
NB that 1 pm = 10 -6 microns = 10 -12 meters
Rare Earth Abundances in Chondrites
•“Sawtooth” pattern of cosmic abundance reflects:
– (1) the way the elements were created
(greater abundances of lighter elements)
– (2) greater stability of nuclei with even atomic numbers
Partition Coefficients for REEs crystal
D melt
(concentration in mineral)
(concentration in melt)
Partition Coefficients for REE in Melts
Amphibole-Melt
D bulk
= X
1
D
1
+ X
2
D
2
+ X
3
D
3
+ … + X n
D n
Chondrite Normalized REE patterns
• By “normalizing” (dividing by abundances in chondrites), the “sawtooth” pattern can be removed.
Trace Element Fractionation
During Partial Melting
La Lu
La
Nd
Sm
Co
Re gion of
Partial Melting
Ni
Rb
Sr
M elting Re sidue
La Lu
From: http://www.geo.cornell.edu/geology/classes/geo302
Differentiation of the Earth
Continental Crust
Rb>Sr
Lu
Nd>Sm
La>Lu
La
Rb>Sr
Nd>Sm
La>Lu
Mantle
(After partial melt extraction)
Rb<Sr
Nd<Sm
La<Lu
La Lu
• Melts extracted from the mantle rise to the crust, carrying with them their “enrichment” in incompatible elements
– Continental crust becomes “incompatible element enriched”
– Mantle becomes “incompatible element depleted”
From: http://www.geo.cornell.edu/geology/classes/geo302
Uses of Isotopes in Petrology
• Processes of magma generation and evolution source region fingerprinting
• Temperature of crystallization
• Thermal history
• Absolute age determination - geochronology
• Indicators of other geological processes, such as advective migration of aqueous fluids around magmatic intrusions
Isotopic Systems and Definitions
• Isotopes of an element are atoms whose nuclei contain the same number of protons but different number of neutrons.
• Two basic types:
– Stable Isotopes: H/D, 18 O / 16 O, C, S, N (light) and Fe, Ag (heavy)
– Radiogenic Isotopes: U/Pb, Rb/Sr, Hf/Lu, K/Ar
Stable Oxygen Isotopes d 18 O‰ = [(R sample
- R standard
)/R standard
] x 1000
Three stable isotopes of O found in nature:
16 O = 99.756%
17 O = 0.039%
18 O = 0.205%
Stable Oxygen Isotopes d 18 O‰ = [(R sample
- R standard
)/R standard
] x 1000
Isotope Exchange Reactions
2Si 16 O
2
+ Fe
3
18 O
4
= 2Si 18 O
2
+ Fe
3
16 O
4 qtz mt qtz mt
This reaction is temperature dependent and therefore can be used to formulate a geothermometer
Radioactive decay and radiogenic Isotopes
• “Radiogenic” isotope ratios are functions of both time and parent/daughter ratios. They can help infer the chemical evolution of the Earth.
– Radioactive decay schemes
• 87 Rb87 Sr (half-life 48 Ga)
• 147 Sm143 Nd (half-life 106 Ga)
• 238 U206 Pb (half-life 4.5 Ga)
• 235 U207 Pb (half-life 0.7 Ga)
• 232 Th208 Pb (half-life 14 Ga)
• “Extinct” radionuclides
– “Extinct” radionuclides have half-lives too short to survive
4.55 Ga, but were present in the early solar system.
87 Rb b –
87 Sr
Half-life and exponential decay
Exponential decay:
Never get to zero!
Linear decay:
Eventually get to zero!
Rate Law for Radioactive Decay
P t
= P o exp (t o
–t)
1st order rate law
Whe re
P
P o t
quan tity of the parent isotope ( i.e.
87
Rb) at tim e t ;
quan tity of the parent isotope at some earlier time t o
, when the isotopic system was closed to any additi ona l is otopic exch ange ;
is the cha racteristic decay constant for the sys tem of interest, which is related to the h alf-li fe, t
1/2
, by th e equa tion below:
ln 2 / t
1/2 t
1/2
is defined as the half-life, wh ich is the amount of tim e requir ed for 1/2 of the origin al parent to decay and is a constant.
Rb/Sr Age Dating Equation
87 Rb t
= 87 Rb o e -
to – t)
(Assume that t = 0, for the p resent)
87 Rb o
+ 87 Sr o
= 87 Rb t
+ 87 Sr t
(Conserva tion of Mass, with conc entration and
87 Sr o
as the initi al
87
Sr t
as the con centration today)
87 Sr t
- 87 Sr o
= 87 Rb t
( e
to – 1)
87 Sr
86
Sr
t
87 Sr
86
Sr
o
87
86
Rb
Sr
t
( e
t
1) y
b
x
m
Rb/Sr Isochron Systematics
M
1
M
2
M
3
Instruments and Techniques
• Mass Spectrometry: measure different abundances of specific nuclides based on atomic mass.
– Basic technique requires ionization of the atomic species of interest and acceleration through a strong magnetic field to cause separation between closely similar masses
( e.g.
87 Sr and 86 Sr). Count individual particles using electronic detectors.
– TIMS: thermal ionization mass spectrometry
– SIMS: secondary ionization mass spectrometry - bombard target with heavy ions or use a laser
– MC-ICP-MS: multicollector-inductively coupled plasma-ms
• Sample Preparation: TIMS requires doing chemical separation using chromatographic columns.
Clean Lab - Chemical Preparation http://www.es.ucsc.edu/images/clean_lab_c.jpg
Thermal Ionization Mass Spectrometer
From: http://www.es.ucsc.edu/images/vgms_c.jpg
Schematic of Sector MS
Zircon Laser Ablation Pit
Mantle-Basalt Compatibility
Parent->Daughter Rb>
Th>
Sr
Pb
U> Pb
Nd>Sm
Hf>Lu
Degree of compatibility
Radiogenic Isotope Ratios & Crust-Mantle Evolution
Continental Crust
Rb>Sr high 87 Sr/ 86 Sr
Nd>Sm low 143 Nd/ 144 Nd
La Lu
Melt same 87 Sr/ 86 Sr and
143 Nd/ 144 Nd as mantle
Mantle
(After partial melt extraction)
Rb<Sr
Nd<Sm low 87 Sr/ 86 Sr
La high 143 Nd/ 144 Nd
Lu
Eventually, parent-daughter ratios are reflected in radiogenic isotope ratios.
From: http://www.geo.cornell.edu/geology/classes/geo302
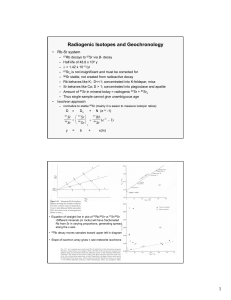
Sr Isotope Evolution on Earth
87 Sr/ 86 Sr)
0
Time before present (Ga)
87 Sr/ 86 Sr)
0
Time before present (Ga)
147 Sm-> 143 Nd
(small->big)
Sr and Nd Isotope Correlations:
The Mantle Array
87 Rb-> 87 Sr (big->small)