Mineral Weathering and Secondary Mineral Formation
advertisement

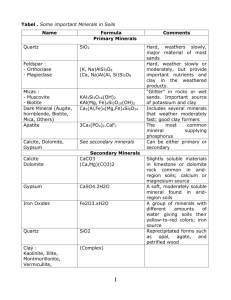
Mineral Weathering and Secondary Mineral Formation weathering: chemical alteration of minerals (in soils, involves water, gases, acids, etc). Parent material soil Desilication via weathering Parent Material=primary silicates formed from igneous/metamorphic processes Soil= secondary silicates, oxides, carbonates, etc.formed from weathering processes Behavior of Elements During Chemical Weathering •Soils are depleted in elements relative to parent material •Element loss/depletion is determined by elements position on periodic table (which column or group of columns) AND the element’s ionic potential Z/R = ionic potential z=charge, r=radius Classes: Z/R= 0-3 ion surrounded by H2O shell, soluble in H2O (Na, Ca, etc) Z/R=3-~9.5 ion so strongly attracts H2O that insoluble oxides/hydroxides form (Al, Fe) Z/R=>~9.5 soluble oxyanions form (S, C, etc.) Ionic potential of important elements •Red arrow indicates decreasing attaction to H2O within a group of elements •Decreasing attraction is reflected in weathering losses….. Element loss varies with ionic potential 2 Ti group N 1.5 C I log (soil Zr /c rust Zr ) Br Alkali metals and alkaline earths 1 0.5 S Zr 0 Ti Li Si A lP Cl -0.5 Fe Rb Sr Tm Nd Dg YLu b uT b Cs P r SEGd Hf m Ba Em Ce Ho Th U KCa Mg F -1 Na Be -1.5 0 20 40 60 atomic number 80 100 Mineral Particle Size and Mineralogy Gravel > 2mm (primary) Sand >= 0.05 to 2.0 (primary) Silt <0.05 to 0.002 (primary + secondary) Clay < 0.002 (secondary) Most secondary mineral are silicates, and most secondary silicates are phyllosilicates. Mineral Classification Tetrahedral Sheet Arrangement Example Chemical Formula of Specific Minerals Si/Al+Fe Phyllosilicates 2 (tetra):1(oc ta) sme ctite group vermi culite group kaolin group silica group iron oxides (montmorilli nite) Mx(Al3.2Fe0.2Mg0.6)(Si 8)O20(OH)4 (1) (trioctahedral vermiculite) Mx(MgFe)6(Si8-xAlx)O20(OH)4(2) (kaolinite) (Al4)(Si 4)O10(OH)8 (opal) SiO2 •nH2O) (3) (geothite) FeOOH 2 (hematite) Fe2O3 0 (ferrihydrite) Fe5(O4H3)3 (4) 0 (gibbsite) Al(OH)3 0 (calcite) CaCO 3 NA NA NA 2 (tetra):1(oc ta) 1 (tetra):1(oc ta) Tectosilicates Oxides NA NA alumi num oxides Carbonates Organic Matter NA NA NA 2 1 infinity 0 CEC (meq(+)/100g mineral 110 (range 47162) (5) 150 (range 144207) (2) 1 (range 0-1) (6) 0 ~0 (pH dependent) (4) ~0 (pH dependent) ~0 (pH dependent) ~0 (pH dependent) ~0 (8) 100-900 (pH dependent) (9) (1) from G. Sposito, The Chemistry of Soils, Oxford University Press, New Yo rk (1989). (2) from L.A. Do uglas, Vermiculites. In: J.B. Dixon and S.B. Weed, Minerals in Soil Environments, 2nd Ed., Soil Science Society of America, Madison, WI (1989). (3) amorphous or paracrystalline (4) from U. Schwertman and R.M. Ta ylor, Iron Oxides, Chap. 8 In: J.B. Dixon and S.B. Weed (op. cit. 2). (5) from G. Borchardt, Smectites, Chap. 14 In: J.B. Dixon and S.B. Weed (op. cit. 2). (6) from J.B. Dixon, Kaolin and Serpentine Group Minerals, Chap. 10 In: J.B. Dixon and S.B. Weed (op. cit. 2). (7) from P.H. Hsu, Aluminum Oxides and Hydroxides, Chap. 7 In: J.B. Dixon and S.B. Weed (op. cit. 2). (8) from H.E. Doner and W.C. Ly nn, Carbonate, Halide, Sulfate, and Sulfide Minerals, Chap. 6, In: J.B. Dixon and S.B. Weed (op. cit. 2). (9) from J.M. Oades, An Introduction to Orga nic Matter in Soils, Chap. 3 In: J.B. Dixon and S.B. Weed (op. cit. 2). Observed Silicate Mineral Weathering Pathways in Soils PRIMARY SILICATES SECONDARY MINERALS NESOSILICATES silicon smectite silicon opal kaolinite iron gibbsite silicon Si(OH)4 INOSILICATES Fe oxides calcium calcite calcium PHYLLOSILICATES biotite muscovite TECTOSILICATES -K trioctahedral illite -K dioctaheral illite trioctahedral vermiculite dioctahedral vermiculite plagioclase feldspars quartz Si(OH)4 INCREASING DEGREE OF DESILICATION 1:1 phyllosilicates: kaolinite •One layer of Si tetrahedra •One layer of Al octahedra •Individual minerals are held to another via H bonds 2:1 Phyllosilicates: di and trioctahedral Dioctahedral (smectites) •Substitution of +2 for +3 in octahedral layer (called isomorphous substitution) •Creates a net negative charge (and property of cation exchange capacity) •Results in expandable layers Trioctahedral (vermiculite) •Substitution of +3 for +4 in tetrahedral layer •Also has CEC, but little or no expansion Other secondary mineral groups: oxides Al oxides (gibbsite) •Results of vigorous chemical weathering (desilication) Non-silicate secondary minerals: oxides Fe oxides 1. Geothite • Yellowish brown • Acidic, OM-rich envir. 2. Hematite • Bright red • Warm, dry environments Non-silicate secondary minerals: carbonates Calcite •Ca is released from some weathering source •Forms in arid to semiarid environments when soil solution becomes saturated •Presence in upper 1m related to MAP •Depth of carbonate layer related to MAP Geographical distribution related to climate •Greater than 100cm/yr removes carbonate •Below 100cm, depth~MAP Non-silicates: sulfates (gypsum) •Presence of sulfates in soils usually occurs in hyperarid climates (or sites with high water table and evaporative enrichment of salts) Secondary Minerals in California Soils: Sierra Nevada Soil Mineralogy vs. Elevation