Electrochemical Cell
advertisement

Experiment #10
Electrochemical Cell
What are the goals of this experiment?
To build a Cu-Zn galvanic cell.
To study the effect of changing concentration
of the electrolytes on the voltage output of the
galvanic cell
Electrochemistry
A study of chemical changes produced by electric
current and with the production of electricity by
chemical reactions.
All electrochemical reactions involve the transfer
of electrons and are therefore oxidation-reduction
reactions.
The reacting system is contained in a cell, and an
electric current enters or exits by electrodes.
Electrochemistry
The sites of oxidation and reduction are separated
physically so that oxidation occurs at one location
while reduction occurs at the other.
Electrons flow from site of oxidation to the site of
reduction. Flow of electrons implies flow of electric
current. Current is usually denoted by symbol “I”
and unit amperes (amps).
Current ‘I’ is related to voltage ‘V’ through, ohms
Law. R is called the resistance.
V IR
Electrochemistry
Cu-Zn galvanic cell
Metallic Cu (s) ----- Electrode
Metallic Zinc (s) ----- Electrode
CuSO4 (aq) --------- Electrolyte
ZnSO4 (aq) --------- Electrolyte
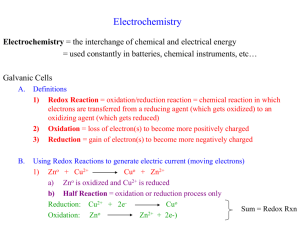
What is oxidation?
Current definition:
Loss of Electrons is Oxidation (LEO)
Na
Na+ + e-
Positive charge represents electron deficiency
ONE POSITIVE CHARGE MEANS DEFICIENT BY ONE ELECTRON
Oxidation occurs at the Anode
What is reduction?
Current definition:
Gain of Electrons is Reduction (GER)
Cl + e-
Cl -
Negative charge represents electron richness
ONE NEGATIVE CHARGE MEANS RICH BY ONE ELECTRON
Reduction occurs at the cathode
Sign conventions in a galvanic cell
A battery has a positive terminal and a
negative terminal
Cathode is assigned a positive (+) sign
Anode is assigned a negative (-) sign
Anodic Oxidation (AO) and Cathodic Reduction
Will this setup sustain flow of electrons?
Reducing half,
will have negative
Charges. Will
have excess anions.
SO42-
Oxidizing half,
will have positive
Charges. Will have
excess cations (Zn2+)
but deficient in anions.
No, this set up does not sustain flow of electrons
due to charge build up.
Will this setup sustain flow of electrons?
We therefore need to provide a pathway for the
anions to flow from the area where they are no longer
needed (where Cu2+ is being converted to neutral Cu)
to the area where they are needed (where neutral Zn is
being converted to Zn2+). In other words, the solutions
must be connected so that ions can flow to keep the
net charge in each compartment zero
How do we connect the two halves?
How do we connect the two halves?
Why do electrons migrate spontaneously
from one electrode to the other?
The electrons move through the wire because by doing
so they will move from a higher-energy to a
lower-energy.
How to identify the oxidizing half-cell
and reducing half-cell?
When two half-cells are connected, the one with the larger
reduction potential (the one with greater tendency to
undergo reduction) acquires electrons from the half-cell
with lower reduction potential, which is therefore forced to
undergo oxidation. The half-cell with the higher standard
reduction potential acts as the cathode (Reduction) and the
half-cell with the lower standard reduction potential acts
as the anode (Oxidation).
What is reduction potential?
It is the voltage that is generated when a
half-cell undergoes reduction.
Where can I obtain these values from?
From the table of
standard reduction potentials
Why is it called
standard reduction potential?
Because these voltages were measured,
when the half-cells underwent reduction
under a set of standard conditions in
combination with a standard electrode.
What is a standard electrode?
It is an electrode, whose reduction/
oxidation potential is known.
standard reduction potential
A reference electrode has been arbitrarily chosen and its
standard reduction potential has been assigned a value of
exactly 0 V. This reference electrode is called the
standard hydrogen electrode.
2 H+(aq, 1.00 M) + 2 e-
H2(g, 1atm)
Standard conditions:
Concentration = 1.0 M
Pressure = 1.0 atm
Figure 5: A galvanic cell composed of copper and hydrogen half-cells
E= 0.00 V
standard reduction potential
E
Units
Volts (V)
standard reduction potential
Increasing tendency to get reduced
Table 1: Given below is a list of some reduction potentials
An Example: What if I were to build
a Cu-Ag galvanic cell?
If we were to build a galvanic cell, we should keep in
mind that, there are two half-cells. One of them
undergoes oxidation and the other undergoes
reduction.
For that we will need to know the values of standard
Reduction potential of each half cell.
Cu2+(aq) +2eAg +(aq) + e-
Cu(s) E = + 0.34 V
Ag (S)
E= + 0.80 V
An Example: What if I were to build
a Cu-Ag galvanic cell?
Cu2+(aq) +2eAg +(aq) + e-
Cu(s) E = + 0.34 V (Oxidizing, Anode)
Ag (S)
E= + 0.80 V (Reducing, Cathode)
So, Ag electrode is the positive(+) electrode and
Cu electrode is the negative (-) electrode.
An Example: How can I find the
Standard reduction potential of the
Cu-Ag galvanic cell?
Cu2+(aq) +2e-
Cu2+(aq) E = + 0.34 V (Oxidizing, Anode)
Ag +(aq) + e-
Ag (S)
E= + 0.80 V (Reducing, Cathode)
standard reduction potential of the half - cell standard reduction potential of the half - cell
E cell
tha
t
undergoes
reduction
th at undergoes oxidation
E
Ag
cell E
Ag
Cu 2
E
Cu
E cell 0.80 0.34 0.46 V
An Example: How can I find the
potential of the Cu-Ag galvanic cell
under any concentration condition?
Cu2+(aq) +2eAg +(aq) + e{Cu(s)
Cu (s) E = + 0.34 V (Oxidizing, Anode)
Ag (S)
E= + 0.80 V (Reducing, Cathode)
Cu2+(aq) + 2e-} ×1
{Ag +(aq) + e-
Ag (S) } × 2
Cu(s) + 2Ag+
Cu2+(aq) + 2Ag
2 e- involved in the
overall process.
Nernst Equation
Ecell
RT
E
ln Q
nF
o
cell
Where Ecell= Voltage measured for the reactant and product concentrations summarized by the
reaction quotient Q;
Ecell= Voltage measured when reactant and product concentrations are 1 M (or 1 atm for
gases)
R = gas constant in units appropriate for the system, 8.314 JK-1mol-1;
T = absolute temperature in K;
n = number of moles of electrons transferred in the oxidation-reduction reaction
F = 96,485 coul/mol e-;
ln Q = natural logarithm of the reaction quotient for the reaction.
Pr oduct of product concentrations
Q
Pr oduct of reac tan t concentrations
Concentration of pure solids are to be omitted.!!
An Example: How can I find the
potential of the Cu-Ag galvanic cell
under any concentration condition?
Cu(s) + 2Ag+
Cu2+(aq) + 2Ag(s)
RT
Ecell E
ln Q
nF
1
2
2
Cu (aq) Ag ( s )
Q
2
1
Cu( s) Ag (aq)
1
2
Cu (aq)
Q
2
Ag (aq)
o
cell
2 e- involved in the
overall process.
An Example: How can I find the
potential of the Cu-Ag galvanic cell
under any concentration condition?
Cu(s) + 2Ag+
Cu2+(aq) + 2Ag(s)
2 e- involved in the
overall process.
RT
Ecell E
ln Q
nF
1
2
RT Cu (aq)
o
Ecell Ecell
ln
2
nF Ag (aq)
o
cell
An Example: How can I find the
potential of the Cu-Ag galvanic cell
under any concentration condition?
RT Cu (aq)
E E
ln
nF Ag (aq)
8.314J
298 K
Cu (aq)
Kmol
E 0.46
ln
Ag (aq)
nmole 96485 Coul
cell
o
cell
2
1
2
cell
mole
0.0591 Cu (aq)
0.46
ln
2
n
Ag (aq)
2
Ecell
1
2
1
2
An Example: How can I find the
potential of the Cu-Zn galvanic cell
under any concentration condition?
1 Cu2+(aq) + 1 Zn(s) 1 Cu(s) + 1 Zn2+(aq)
RT
Ecell E
ln Q
nF
1
1
2
Cu ( S ) Zn (aq)
Q
1
1
2
Cu (aq) Zn( s)
1
2
Zn (aq)
Q
1
2
Cu (aq)
o
cell
An Example: How can I find the
potential of the Cu-Zn galvanic cell
under any concentration condition?
RT Zn (aq)
E E
ln
nF Cu (aq)
8.314J
298 K
Zn (aq)
Kmol
E E
ln
Cu (aq)
nmole 96485 Coul
cell
cell
o
cell
2
1
2
1
cell
Ecell
mole
1
2
0.0591 Zn (aq)
E
ln
1
n
Cu (aq)
2
1
2
1
An Example: How does the voltage output of
the Cu-Zn galvanic cell change with change in
concentration of Cu2+(aq) and Zn2+(aq)?
0.0591 Zn (aq)
E
ln
1
n
Cu (aq)
2
Ecell
[Zn2+(aq)] (M)
1
[Cu2+(aq)] (M)
2.00
1.00
1.00
1.00
1.00
2.00
Ecell (V)
Ecell – 8.89 10-3
Ecell
Ecell + 8.89 10-3
Standard free energy change (G)
and Free energy change G)
Free energy in this context refers to the energy that
is generated by the movement of electrons for doing
work.
Stands for change
G stands for Gibbs free energy
G qEcell n F Ecell
G qEcell n F Ecell
Units of free energy change
G n
F
E cell
coulombs joule
(mole e )(
)(
) joule
mole e- coulomb