Stem Cell Research - Financial Analysis Office
advertisement

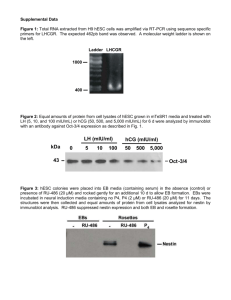
STEM CELL RESEARCH: GETTING ALL THE DUCKS IN A ROW Sara Bible, Dean of Research Office Stanford University Bill Brophy, Director of Financial Analysis University of California, San Diego Presented at the SRA Western Regional Meeting RESEARCH ELEVATED: MILE HIGH OPPORTUNITIES DENVER, CO - JUNE 7-11, 2008 About This Session TOPICS Impact of federal funding issues on administration of hESC research Human Embryonic Stem Cell Research Special Operating Procedures for: material transfer agreements accounting effort allocation and certification facilities equipment expendable materials and supplies derivatives research data and intellectual property About This Session ABOUT THIS SESSION Session based on a tutorial for Stanford personnel, including faculty, staff, postdoctoral scholars, students, visiting scholars and other researchers, who will be working on research projects involving human embryonic stem cells. At Stanford, all personnel must complete this tutorial satisfactorily as a prerequisite to beginning work or study associated with human embryonic stem cells. It is the PI’s responsibility to ensure that personnel complete both tutorials. Tutorial includes post test that must be completed with 100% accuracy. At UCSD, Research personnel with responsibilities for research using Human Embryonic Stem Cells are required to complete training in stem cell research, policy, and ethics. STEM CELL RESEARCH TERMS AND ACRONYMS Human Adult Stem Cells Stem cell = master cell that can create the more specialized human cells Human Embryonic Stem Cells (hESC) Pluripotent cells that are self-replicating, derived from human embryos and are capable of developing into cells and tissues of the three primary germ layers. Although human embryonic stem cells may be derived from embryos, such stem cells are not themselves embryos. Registry Lines/Registry hESC Those included on the NIH Human Embryonic Stem Cell Registry Non-registry Lines/Non-Registry hESC Those excluded from the NIH Human Embryonic Stem Cell Registry hESC lines A population of identical cells that all originated from a single blastocyst. Because cell lines multiply, scientists can grow the cells for their own research and to share with colleagues. CRITERIA FOR hESC RESEARCH FEDERAL FUNDING Federal Announcement On August 9, 2001, President George W. Bush announced that federal funds can be used for hESC research only if they meet the following criteria: 1. The stem cells were derived from an embryo that was created for reproductive purposes and was no longer needed; 2, informed consent was obtained for the donation of the embryo, and the donation did not involve financial inducements; and 3. the process of derivation was begun prior to 9 pm EDT on August 9, 2001. Please think of your own preferred quotation for this picture. FEDERAL ANNOUNCEMENT IMPACTS FEDERAL FUNDING FOR hESC RESEARCH Because of President Bush’s announcement, hESC lines are categorized as: 1. Registry hESC Lines Federal funds may be used ONLY for research using registry hESC lines. Registry hESC lines = included on the NIH Human Embryonic Stem Cell Registry - confirms that the stem cell lines are in compliance with the President’s criteria as stated on August 9, 2001. 2. Non-registry hESC Lines Federal funds may not be used directly or indirectly for research using nonregistry hESC lines (or their derivatives). Non-registry hESC lines are excluded from the NIH registry because they do not meet federal criteria. hESC Research Can Proceed Although the August 9, 2001 announcement bars the use of federal funds for research on non-registry hESC lines, it does not prevent investigators from conducting research with non-federal funds. GOAL Implement Procedures: to ensure hESC research can proceed unimpeded, to ensure compliance with university, federal and state policies, laws and regulations, and to ensure federal funds are not used to support non-registered hESC research directly or indirectly. to ensure all faculty, staff, postdoctoral scholars, and students as well as visiting scholars and other researchers involved in hESC are trained. ADDITIONAL RESPONSIBILITIES INSTITUTIONAL RESPONSIBILITIES • Review all human stem cell protocols • Review ownership and use restrictions for all equipment in the space • Review and negotiate agreements for obtaining hESC lines • Tag equipment accurately based on federal funding restriction • Monitor space and facilities used for research on non-registry hESC • Perform indirect cost calculation, and verify funding source of labs ADDITIONAL RESPONSIBILITIES RESPONSIBILITIES OF RESEARCH PERSONNEL • Plan the Research – where, who, what (funding, equipment, cell lines) • Review campus guidelines, complete hESC, cell culture and ethics training • Obtain approval of research protocol or amendment and material transfer for each hESC line requested. • Establish appropriate accounting for each research activity. • Obtain institutional biosafety approval where needed COSTING PROCEDURES: INTRODUCTION • The following costing procedures provide guidance to ensure that federal funds are not used to support nonregistry hESC research. • Remember that the costing procedures for hESC research are in addition to existing institutional policy & procedures. STANFORD PROCESS Stem Cell Research Tracking (SCRT) Form Form must be completed for all research that involves use of all Human Stem Cells - registry and non-registry hESC or human embryos regardless of funding source - gifts, departmental funds, university research or externally funded grants and contracts. When Must the Form Be Completed? 1. At the time of proposal (for an externally - funded project) 2. 30 days prior to anticipated start date (for gift, department or university research funded project) 3. Whenever there is a change in project location/space usage such as room additions or deletions. Purpose of the Form • Inform and seek approval from cognizant personnel • Begin Stem Cell Oversight Committee (SCRO) review of scientific and ethical issues • Alert PMO & your DPA so they can assess ownership of the equipment to be used in research. • Initiate accounting – to open a Project Task Award (PTA). UCSD PROCESS Before research/teaching w/human embryonic stem cells, must be approved by: • Embryonic Stem Cell Research Oversight Committee (ESCRO) http://research.ucsd.edu/escro • UCSD Human Research Protections Program IRB http://irb.ucsd.edu • Institutional Biosafety Committee - EH&S http://www-ehs.ucsd.edu • Conflict of Interest (IRC) http://ocga3.ucsd.edu If project calls for use of animal subjects, must also be approved by: • Institutional Animal Care and Use Committee (IACUC) http://iacuc.ucsd.edu If human embryonic stem cells provided by non-UCSD source, must have: An MTA - Office of Contract and Grant Administration http://ocga3.ucsd.edu, OR A Purchase Agreement - UCSD Purchasing http://www-bfs.ucsd.edu/pur UCSD PROCESS If UCSD-developed cells are sent outside UCSD or transferred between UCSD investigators, must receive an evaluation from: • Technology Transfer - TechTIPS http://invent.ucsd.edu Research w/human embryonic stem cells, non-UCSD fund source, must be approved by: • ESCRO, and campus IRB • Office of Contract and Grant Administration http://ocga3.ucsd.edu OR • Clinical Trials Administrative Services & Research Compliance Office If research or training is funded by department or gift funds, approval is required from the department business office and/or department chair. ACCOUNTING STANFORD New PTA Required Open a new Project Task Award (PTA) for each project utilizing non-registry human embryonic stem cells, whether it is gift, departmental university, or externally funded research. A research assistant prepares stem cell cultures in a lab at the Waisman Center. Photo by: Jeff Miller, UWM-2001 ACCOUNTING UCSD Establish separate fund for each award - set up index, fund, and other accounting elements that drive University's reporting/ledger processes Prepare and manage award allocations Approve financial transactions to be charged to the project Ensure that award expenditures comply w/financial terms and conditions, University/agency policies EFFORT ALLOCATION AND CERTIFICATION Use established policies for: • tracking, allocating and confirming effort on sponsored projects for all personnel to ensure that effort devoted to non-registered hESC research is not paid with federal funds, • monthly review and quarterly certification of expenditure statements, by the Principal Investigator, • proper allocation of salaries and benefits when an individual is working with multiple sources of support. Important! Salary must be allocated and charged to gift and departmental, or university accounts commensurate with effort put forth on the projects! EFFORT ALLOCATION AND CERTIFICATION . Special Operating Procedures for additional work involving hESC research for postdoctoral fellows and students funded 100% by federal sources. Postdoctoral fellows and students who are funded by federal institutional training grants or fellowships, such as National Research Service Awards (NRSA) are required to pursue their research training full time under the terms of the award. However, they may be permitted under the sponsor’s policy to engage in limited or part-time work beyond their federal commitments. Any such additional work must be compensated as part time employment and must be incidental to the training program. USE OF UNIVERSITY AND FEDERAL FACILITIES Federal funded facilities, including VA facilities, may not be used in the conduct of non-registry hESC research. Both Stanford and UCSD have identified their federally funded facilities and prohibit their use for non-registry hESC research. FACILITIES FOR NON-REGISTRY hESC RESEARCH Where research on non-registry hESC is conducted • List each lab, room, and office that will be used. Obtain a determination on whether any of the proposed spaces cannot be used because of federal funding. • Review all equipment in the proposed spaces to see if any have been tagged as federally funded. If so, the items cannot be used on the non-registry research project. • Update and re-route the form before the project location changes. SPACE INVENTORY CODING Both Stanford and UCSD use their established space inventory and inventory procedures to track the location of all hESC research. • Code space as organized research or departmental research, depending on the funding of the research. • Use the Research Explanation or Comments fields to document non-registry hESC research EQUIPMENT APPROVAL - STANFORD In a facility where non-registry hESC research is performed, all new and existing equipment regardless of funding source, must be reviewed for federal funding restrictions, and tagged appropriately. It will be tagged as either APPROVED for use on non-registry hESC or DO NOT USE on non-registry hESC. Equipment is considered to have a purchase price of $5,000 or more and a useful life of more than one year. • Develop a list of all equipment in a facility where non-registry hESC research will be performed. Once the list has been Tags for Equipment where completed, subsequent purchases will be non-registry hESC is reviewed individually. conducted APPROVED DO NOT USE FOR USE ON NON-REGISTRY hESC ON NON- REGISTRY hESC • Will verify ownership and use restrictions. • Approval to use the equipment may or may not be granted. • All Equipment will be tagged to indicate whether it is approved or not approved for non-registry hESC research. EQUIPMENT APPROVAL - UCSD In 2007, an inventory was conducted of all academic equipment, to identify current building and room location. A red dot was affixed to all federally funded equipment, and a campus procedure was published and disseminated, indicating that red dot equipment could not be used for non-registry hESC research. Capital Assets Accounting issues a red dot along with an inventory tag for all equipment purchases that are federally funded. EQUIPMENT OWNERSHIP CATEGORIES Equipment ownership will fall into one of three categories and will be tagged accordingly. Equipment owned by the federal government Equipment purchased with federal funds may be owned by the University and therefore can be used for non-registry hESC research, if one of the following criteria is met: DO NOT USE ON NON-REGISTRY hESC All competitive segments of the federal grant or contract supporting the equipment purchase has been completed, University retains title to the equipment without restriction; Or University purchases the equipment in full, without federal restriction, and can document such transaction. Equipment owned by the University APPROVED FOR USE ON NON-REGISTRY hESC EQUIPMENT INVENTORY CONDUCTED PERIODICALLY Laboratories where non-registry hESC research is performed are subject to periodic inventories to ensure that only approved equipment is used to support non-registry hESC research. Additional detail on Stanford procedures for equipment when conducting hESC research can be found in the Property Administration Manual, Chapter 14 “Using Equipment for Stem Cell Research”. MATERIALS/SUPPLIES ALLOCATION METHODOLOGY If you purchase expendable materials and supplies that benefit multiple projects, use a reasonable allocation methodology to accurately assign costs to the benefiting projects. This allocation methodology must be documented. When making such purchases to support non-registry hESC research, make certain costs are not allocated to federal projects. To avoid risk of charging materials and supplies for non-registry hESC to federal projects, purchase supplies for non-registry hESC research separate from all other research where practical. SERVICE CENTERS Service Centers used for research with non-registry hESC must not involve any federal resources or federal core funding. If a service center includes equipment that is federally funded and title remains with the federal government, some additional steps may be needed to allow use to provide services to research with non-registry hESC, including an increased charge rate to recognize the depreciation of the federal equipment, and a designation of program income for the income so produced. DERIVATIVES FROM hESC RESEARCH Research using derivatives from non-registry hESC lines may not be supported by federal funds. For costing purposes they are treated just like the lines themselves. Use the appropriate campus procedure to record: 1. Source of the hESC lines and the hESC derivatives, 2. Whether the source is a registry line or non-registry line, 3. Funding source of your research. Caption: Derived from human embryonic stem cells,in the lab of UW-Madison stem cell researcher Su-Chun Zhang, 11/01 MATERIAL TRANSFER AGREEMENTS Follow institutional policies for receiving research materials. All hESC lines require a Material Transfer Agreement (MTA) or similar agreement in place between the provider and University before the lines can be transferred to investigators Investigators requesting hESC lines, both registry and non-registry, must fill out an MTA routing form. The provider's agreement for transferring the cell lines will be reviewed to ensure compliance with University policy; necessary changes will be negotiated before the agreement is signed. Stanford’s Instructions for obtaining hESC lines can be found at the ICO's website: http://www.stanford.edu/group/ICO/agmts/index.htm DATA & INTELLECTUAL PROPERTY Researchers may use data or information obtained from either registry or non-registry hESC research in subsequent hESC projects if: 1. the data has been published or 2. permission to use the data has been granted by the original researcher whether the subsequent projects are federally or non-federally funded. However, the federal government may not be charged for generating data from non-registry hESC research, or for analyzing or manipulating data for subsequent use in non-registry hESC research. Data and information usage is subject to the usual considerations of thirdparty intellectual rights the as well as any specific grant or contract conditions on data usage imposed by suppliers or sponsors of the data, including other research institutions and federal funding agencies.