Matter - St. Paul School
advertisement

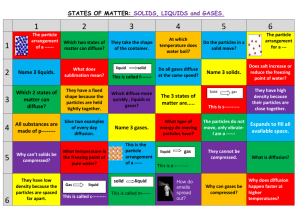
Matter Section 3.1 What do you know? Where can you find matter? Do you think there is any place where you wouldn’t find matter? How are liquids different from gases? What are some names for the particles of matter? http://www.sfu.ca/~sjl12/iat334/asg3/img/question%2520mark.jpg Can You Read My Mind? 1. Mentally, select an object in your classroom. 2. Write everything you can think of to describe the object without actually naming it. 3. How many different ways can you describe the object? 4. See if a classmate can guess what your object is, based only on your descriptions. http://i.ytimg.com/vi/fIC4FDfCxk0/maxresdefault.jpg Matter Matter- anything that takes up space and has mass Mass- amount of matter in an object. Mass of an object is not the same as the space it takes up. Everything is made of matter including the entire universe. http://www.ridge.k12.wa.us/cms/lib01/WA01000666/Centricity/Domain/121/Poperties%20of%20Matter.png Physical Properties of Matter Physical properties- things that can be observed or measured without changing the makeup of a substance. Mass, volume, and density are physical properties of matter. Each different kind of matter has different physical properties. Phases of Matter All matter is made of tiny particles. From observations they have developed the particle theory of matter (Observations in Table 3.1). What causes particles to move faster? 3 Phases of Matter: Solids Liquids Gases http://sciencewithme.com/wp-content/uploads/2010/11/states_matter_1.jpg Solids Definite shape Definite volume Particles are close Particles vibrate back and forth but held in fixed positions by strong forces. http://www.middleschoolchemistry.com/img/content/multimedia/chapter_1/lesson_5/solid.jpg Liquids Definite volume Does not have definite shape Particles move freely Liquid takes shape of container it is in. http://www.middleschoolchemistry.com/img/content/multimedia/chapter_1/lesson_5/liquid.jpg Gases Does not have definite shape. Does not have definite volume. Particles move constantly and rapidly in all directions. Particles are far apart. Particles fill all available space in a container. http://www.middleschoolchemistry.com/img/content/multimedia/chapter_1/lesson_5/gas.jpg Questions How do the particles in solids, liquids, and gases differ? How does the shape of the container affect liquids and gases? How does shape and volume affect solids, liquids, and gases? Look at Figure 3.2 on page 52: Which drawing shows the fastest-moving particles? Which drawing shows particles arranged in a pattern? How would you use the drawings to explain why gases tend to be less heavy than solids? http://www.aumethodists.org/wp-content/uploads/2011/01/question-marks.jpg Elements Elements- a form of matter that cannot be changed into simpler substances by any chemical process or by heating. Exist in nature as solids, liquids, or gases. Most elements are solids at room temperature. Elements that are present as gases are in the earth’s atmosphere. (they are odorless and colorless) Only two elements exist as liquid at room temperature: mercury and bromine. http://scienceblogs.com/startswithabang/files/2014/03/PeriodicTableBlack.png Atoms Atoms- building blocks of elements Atoms combine with other atoms to make molecules Each kind of atom has properties that make it different from the atoms that make up other elements. Each atom is made up of different particles called subatomic particles. Proton- positive charge; in the nuclues Neutron- no charge; in the nucleus Electron- negative charge; outside of nucleus http://i1.wp.com/www.universetoday.com/wp-content/uploads/2010/02/c-atom_e1.gif Compounds and Molecules Compound- substance that is made of more than one kind of element. Compounds are formed from elements combining chemically. Most are formed from molecules. Molecules- smallest part of a compound that has all the properties of the compound; two or more atoms chemically bonded. Elements that make up a compound are always present in the same proportions. A compound cannot be separated into the elements that form it except through a chemical reaction. http://vignette4.wikia.nocookie.net/powerlisting/images/0/0d/1022molecule.jpg/revision/latest?cb=20110813211337 Chemical Symbols and Formulas Chemical Symbol- the shorthand name of an element, consisting of either a single capital letter or a capital and lowercase letter. Chemical Formula- a combination of chemical symbols that represent a compound. Sometimes a formula represents a molecule of an element, not a compound. (eg. N2 diatomic molecule) Sometimes a molecule has a subscript which indicates the number of atoms in the molecule. http://91ef69bade70f992a001-b6054e05bb416c4c4b6f3b0ef3e0f71d.r93.cf3.rackcdn.com/chemical-symbols-showing-chemistry-andscience-100207056.jpg Common elements in living things 6 most abundant elements in living things. Oxygen Carbon Hydrogen Nitrogen Sulfur Phosphorus What have we learned? How does an atom differ from a molecule? The chemical formula for calcium carbonate is CaCO3. What elements make up CaCO3? How many atoms are in one molecule of CaCO3? Rust is a substance that forms when iron combines with oxygen. Is rust an element or a compound? What are the six most abundant elements in living things? http://adifferentmirror.thebodynarratives.com/wp-content/uploads/2014/04/stock-illustration-5853965-question-mark.jpg