Chem_Test_Outline[1]
advertisement
![Chem_Test_Outline[1]](http://s2.studylib.net/store/data/010130217_1-9c615a6ff3b14001407f2b5a7a2322ac-768x994.png)
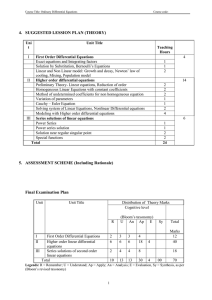
SNC 2D1 - Chemistry Unit Test Outline: o Will be given a periodic table WITHOUT names/charges. o NO polyatomic table will be given (memorize the seven from our note: ammonium, hydroxide, nitrate, carbonate, phosphate, chlorate, sulfate) o Lewis Dot Diagrams (draw) o Atoms vs. Ions (difference and write in standard form) o Ionic Bonds vs. Covalent Bonds o Naming Compounds (symbols name and name symbols): - Ionic (“normal”, Polyatomic, Multivalent) (“criss-cross rule”) - Molecular (mono/di/tri/tetra/penta/hexa) ** Remember the “Diatomic 7” (HOFBrINCl) & combining capacity of Silver (1+) & Zinc (2+) o Reactants, Products, Word Equations, Skeleton Equations and Balanced equations (know how to go from word equation to chemical equation and vice versa) o 3 states (s,l,g) + aqueous (not in equations, just knowledge) o Law of Conservation of Mass (does the mass change in reactions?) o Balancing Equations o Types of Reactions (recognize from equation) o Corrosion (benefits/problems, rust? How to prevent (Galvanizing, material choice, etc.). o Predicting Products (need to know Types of Reactions and exceptions such as “metal oxide + water ” … see note) o Acids & Bases: Differences (Table), Chemical Formulas, Indicators, Neutralization Reactions, common examples/uses, pH Scale ** Test will be ~30 Multiple Choice/Matching & “Short Answer” questions (naming, balancing, application questions, etc) SNC 2D1 - Chemistry Unit Test Outline: o Will be given a periodic table WITHOUT names/charges. o NO polyatomic table will be given (memorize the seven from our note: ammonium, hydroxide, nitrate, carbonate, phosphate, chlorate, sulfate) o Lewis Dot Diagrams (draw) o Atoms vs. Ions (difference and write in standard form) o Ionic Bonds vs. Covalent Bonds o Naming Compounds (symbols name and name symbols): - Ionic (“normal”, Polyatomic, Multivalent) (“criss-cross rule”) - Molecular (mono/di/tri/tetra/penta/hexa) ** Remember the “Diatomic 7” (HOFBrINCl) & combining capacity of Silver (1+) & Zinc (2+) o Reactants, Products, Word Equations, Skeleton Equations and Balanced equations (know how to go from word equation to chemical equation and vice versa) o 3 states (s,l,g) + aqueous (not in equations, just knowledge) o Law of Conservation of Mass (does the mass change in reactions?) o Balancing Equations o Types of Reactions (recognize from equation) o Corrosion (benefits/problems, rust? How to prevent (Galvanizing, material choice, etc.). o Predicting Products (need to know Types of Reactions and exceptions such as “metal oxide + water ” … see note) o Acids & Bases: Differences (Table), Chemical Formulas, Indicators, Neutralization Reactions, common examples/uses, pH Scale ** Test will be ~30 Multiple Choice/Matching & “Short Answer” questions (naming, balancing, application questions, etc)