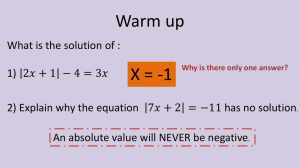Lesson 9
advertisement

Lesson 9 Word Equations Chemical reactions are often represented using word equations. A word equation describes the reactants and products of a chemical equation using the names of the chemicals. Turn to page 42 of the textbook. Read the introductory information in "Word Equations." Then read "Writing Word Equations." This will summarize what you have learned about chemical reactions thus far, and it will provide an introduction to writing word equations. 1. List the three things you have learned thus far about chemical reactions. 2. Why do chemists use word equations to represent chemical reactions? 3. Describe the standard format for writing a word equation for a chemical reaction. 4. What is the purpose of the plus sign (+) in a word equation that represents a chemical reaction? You are already familiar with a number of commonly occurring chemical reactions, such as the reaction for cellular respiration. Read "Word Equations for some Chemical Reactions" on page 43 of the textbook. Familiarize yourself with the word equations for some commonly occurring chemical reactions. 5. a. Write the word equation for the process of cellular respiration. b. Write out how you read this equation? c. How many reactants and products are there in the word equation? 6. a. Write the word equation for the reaction that makes caves in areas of limestone. b. How many reactants and products are in this equation? 7. a. Write the word equation for the addition of hydrochloric acid to mossy zinc. b. How does the word equation describe what you would observe if you actually added hydrochloric acid to mossy zinc?