Food Chemistry - My Teacher Pages
advertisement

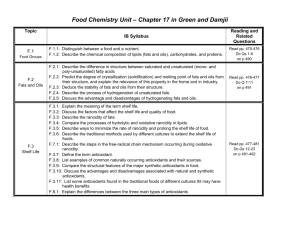
Food Chemistry Chapter 17 in Green / Damjii Chapter X in Nuess Chapter X in Oxford Study (no resource in Chang) F.3: Shelf Life Homework • Read F3 – Shelf Life - pp. 477-481 • Do Qs 12-23 • on p 491-492 F.3.1: Explain the meaning of the term shelf life. Length of time a product can be stored without degradation* of the food item to the point that it is undesirable or unfit for consumption. * change in flavor, smell, texture, and appearance or growth of organisms F.3.2: Discuss the factors that affect the shelf life and quality of food. 1. CHANGE in WATER CONTENT • Moist foods lose water • • • Food becomes dry Change in texture Inc oxidation due to inc exposure to air – this … – – – • dec nutrient value Discoloration of surface Rancidity Dry foods gain water • • • Food becomes moist Change in texture More vulnerable to microbial degradation F.3.2: Discuss the factors that affect the shelf life and quality of food. 2. Chemical Reaction • Chemical changes within food may produce • • • • pH changes (lead to sour taste) formation of other chemicals with undesirable flavors color changes decrease in nutritional value 3. Light • Provides energy for photochemical reactions • • • Rancidity Color fading Oxidation of nutrients – esp vitamins F.3.2: Discuss the factors that affect the shelf life and quality of food. 4. Temperature • Inc in temp = inc in reaction rate 5. Combination of factors • Inc in temp and/or change in pH tends to disrupt forces (bonds & IMFs) holding protein and carbohydrate groups together… this tends to reduce the amount of water that type of food can hold… which then impacts texture, softness, etc. F.3.3: Describe the rancidity of fats. Rancidity: development of unpleasant smells in fats and oils… often accompanied by changes to texture and appearance. Can be… 1) Hydrolytic 2) Oxidative F.3.4: Compare the processes of hydrolytic and oxidative rancidity in lipids. 1) Hydrolytic • Break ester bond - requires − Lipase (an enzyme) [often produced by microorganisms] − Heat [i.e. deep frying] − Moisture (water) • Forms fatty acids with unpleasant odors / taste – – Butanoic acid ; hexanoic acid; octanoic acid In milk / butter • Forms fatty acids that change texture – – palmitic acid ; stearic acid; oleic acid in chocolate / cocoa butter F.3.4: Compare the processes of hydrolytic and oxidative rancidity in lipids. 2) Oxidative (auto-oxidation) • Reaction of carbon-carbon double bond with oxygen from air… using a free radical mechanism. • This requires… − Unsaturated lipid (i.e fish oils) − Oxygen • Is enhanced by… – – Light (helps produce the free radicals) Enzymes (usu produced by micro-organisms) • Forms a wide variety of products – many with unpleasant odors / taste F.3.4: Compare the processes of hydrolytic and oxidative rancidity in lipids. F7.1: Describe the steps in the free-radical chain mechanism occurring during oxidative rancidity. Mechanism for Oxidative Rancidity: (p 479) • Initiation (w/ light; homolytic fission) • Propagation • Termination F.3.5: Describe ways to minimize the rate of rancidity and prolong the shelf life of food. 1) PACKAGING • Opaque, colored glass bottles – reduce exposure to light • Gas-impermeable wrapping film – reduce exposure to oxygen and water • Vacuum packaging – reduce exposure to oxygen and water • fill package with inert gas – i.e. nitrogen F.3.5: Describe ways to minimize the rate of rancidity and prolong the shelf life of food. 2) STORAGE • Refrigeration – • In the Dark – limit access to light – • reduce rate of reactions reduce rate of photo-oxidation Reduce Water Level by Drying or Smoking – – reduce rate of hydrolytic rancidity discourage growth of micro-organisms F.3.5: Describe ways to minimize the rate of rancidity and prolong the shelf life of food. 3) ADDITIVES • Salting reduces water content (osmosis) – • High Sugar content reduces water content (osmosis) – • (preserves) Sulfur dioxide & Sodium Sulfites are reducing agents that prevent oxidation reactions that cause browning – • (bacon) (fruit products) Sodium and Potassium Nitrites and Nitrates are reducing agents that prevent oxidation reactions – (curing meats) F.3.5: Describe ways to minimize the rate of rancidity and prolong the shelf life of food. 4) ANTI-MICROBIAL AGENTS • Discourage growth of micro-organisms in food • • Pickling – vinegar makes environment too acidic for micro-organisms Organic acids and their salts – – • Benzoic acid / sodium benzoate added to fruit juices Propanoic acid / sodium & calcium salts added to bread and cheese Fermentation produces ethanol which limits growth of bacteria – Wine keeps better than juice; distilled spirits keep better than wine (higher % alcohol) F.3.6: Describe the traditional methods used by different cultures to extend the shelf life of foods. • • • • • • Fermentation Pickling Salting Preserves Drying Smoking F.3.6: Describe the traditional methods used by different cultures to extend the shelf life of foods. • Fermentation – Beer “beer is stabilized…beer is one of the first beverages identified that does not readily spoil…beer is stabilized by the fermentation process…beer becomes resistant to action by many other microorganisms…particularly true when air is excluded from the beer by storage in air-tight containers.” – Cheese Fermentation can lower pH to a level that inhibits bacterial and enzyme activity… for instance, in the production of cheese, lactobacilli are added as a starter to change the lactose (milk sugar) into lactic acid… the resulting lower pH inhibits further deterioration F.3.6: Describe the traditional methods used by different cultures to extend the shelf life of foods. • Pickling – Gherkins, Onions Addition of vinegar retards growth of bacteria and fungi Vinegar = %5 ethanoic acid… undissociated acid is responsible for inhibition of microbial growth – Yogurt Contains lactic acid • Salting – Salt is added to food (one of the oldest methods of preserving) Salt dissolves… makes solution… bacteria in contact with solution lose water to the solution via the cell membrane through osmosis… cells dehydrate… incapable of cell division F.3.6: Describe the traditional methods used by different cultures to extend the shelf life of foods. • Preserves – Jams, Condensed milk high concentration of sugar to a food substance Sometimes the surface of these foods collects additional water, reducing the concentration of the sugar, allowing the growth of molds • Drying – Herbs, fruits, meats, and vegetables Removes water… one material that tends to reduce shelf life F.3.6: Describe the traditional methods used by different cultures to extend the shelf life of foods. • Smoking – Meats, Fish Warm, smoke-containing air is passed over food and food gets covered with condensed tar (mostly phenol and aldehydes) Wood smoke contains well over 200 compounds – including ethanol, ethanal, methanol, methanal methanal is the major microbial • Irradiation – Crops, processed foods Beta and gamma radiation are used to irradiate food Inhibit deterioration processes, destroy bacteria and insects May also destroy vitamins and denature proteins May produced undesirable changes and impact eating quality F3.7: Define the term antioxidant. Antidoxidant – substance that can be added to food to increase its shelf life by delaying the onset of oxidative degradation. React with oxygen-containing free radicals… preventing degradation F.3.8: List examples of common naturally occurring antioxidants and their sources. Antioxidant Source Vitamin C (ascorbic acid) Citrus fruits, green leafy vegetables, green peppers, broccoli, strawberries, raw cabbage, potatos Vitamin E (α-tocopherol) [main lipid soluble antioxidant] Carrots, squash, broccoli, sweat potatoes, tomatoes, kale, cantaloupe, peaches, apricots, nuts, seeds, soy beans, whole grains, some vegetable oils (canola oil) Β-carotene Vegetables – carrots & broccoli Fruits – tomatoes & peaches Selenium Trace element found in fish, shellfish, meat, eggs, chicken, garlic and grains F.3.11: List some antioxidants found in the traditional foods of different cultures that may have health benefits. Other foods rich in anti-oxidants include: • green tea • Turmeric • oregano • Blueberries • Cranberries • dark chocolate F.3.11: List some antioxidants found in the traditional foods of different cultures that may have health benefits. Other foods rich in anti-oxidants include: • green tea • Turmeric • oregano • Blueberries • Cranberries • dark chocolate F.3.9: Compare the structural features of the major synthetic antioxidants in food. Common SYNTHETIC antioxidants include: • BHA: 2- and 3-tert-butyl-4-hydroxyanisole • BHT: 3,5-di-tert-butyl-4-hydroxytoluene • PG: propyl gallate • THBP: tertbutylhydroquinone Almost all of these have • Phenolic type structure – alcohol group (hydroxyl) attached to benzene • This structure is NOT found in natural anti-oxidants F.3.10: Discuss the advantages and disadvantages associated with natural and synthetic antioxidants. Natural Antioxidants Advantages Synthetic Antioxidants May reduce risk of cancer and More effective than natural antiheart disease oxidants Some are precursors to other important molecules… Vitamin C for hormones and collagen ; β-carotene for Vitamin A May boost immune system and combat aging and agerelated diseases Diet high in fruit and vegetables also means diet high in fiber and lower in fat - F.3.10: Discuss the advantages and disadvantages associated with natural and synthetic antioxidants. Natural Antioxidants Disadvantages Less effective than synthetics Can be expensive; may add color and aftertaste to foods; too much may actually be harmful Synthetic Antioxidants Safety not yet ‘proven’ Are food additives… and therefore require strictly enforced codes to ensure safety … difficult to police in developing countries High levels of antioxidants may lead to a bitter taste (i.e. some green leafy vegetables) Excessive suppression of oxidation may not be healthy as some toxic free radical mechanisms are used by some cells in the immune system F.8.1: Explain the differences between the three main types of antioxidants. 1) Free Radical Quenchers Species that react with free radicals to produce less reactive free radicals • Natural free radical quenchers – • Vitamin E (tocopherol) Synthetic free radical quenchers – – – BHA TBHQ BHT F.8.1: Explain the differences between the three main types of antioxidants. 2) Chelating Agents Form stable complex ions with transition metal ions… reducing the opportunity for the transition metals to react with hydroperoxides to form more radicals • Many plants contain natural chelating agents – • Rosemary, tea, mustard Synthetic chelating agent – Salts of EDTA (ethylenediaminetetraacetic acid) F.8.1: Explain the differences between the three main types of antioxidants. 3) Reducing Agents (electron donors) react with oxygen in food – decreasing oxidation of food react with hydroperoxides initially produced by auto-oxidation – thereby decreasing free radicals • natural reducing agents – • ascorbic acid & carotenoids synthetic reducing agents – sulfur dioxide, sulfites, nitrites