Unit 2 Notes
advertisement
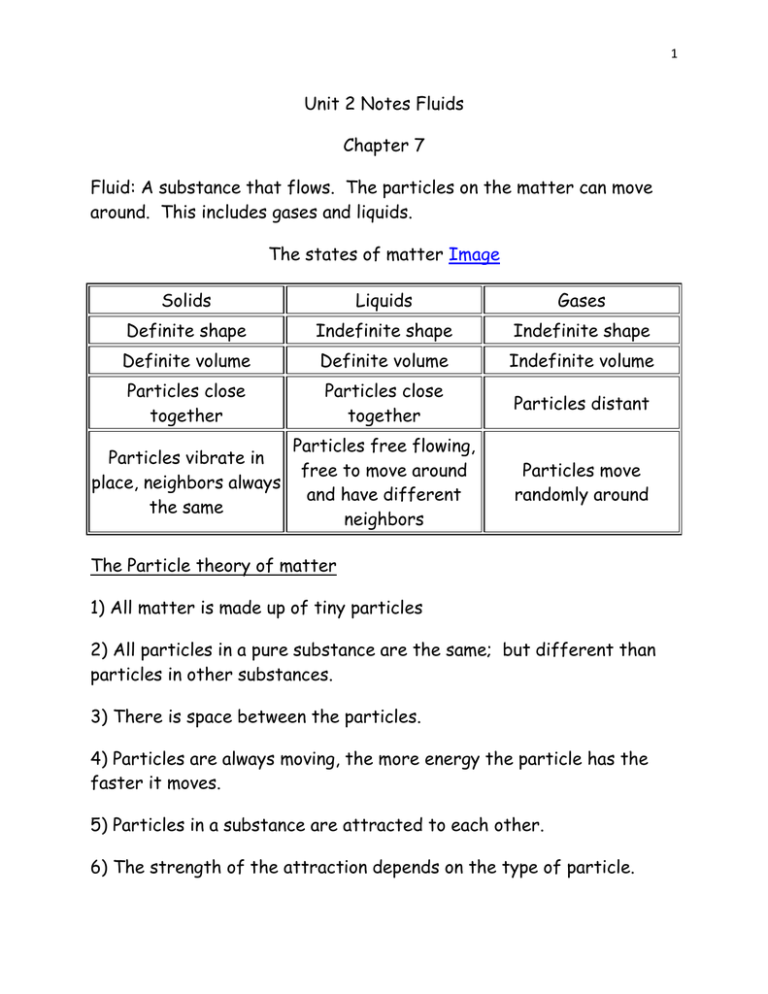
1 Unit 2 Notes Fluids Chapter 7 Fluid: A substance that flows. The particles on the matter can move around. This includes gases and liquids. The states of matter Image Solids Liquids Gases Definite shape Indefinite shape Indefinite shape Definite volume Definite volume Indefinite volume Particles close together Particles close together Particles distant Particles vibrate in place, neighbors always the same Particles free flowing, free to move around and have different neighbors Particles move randomly around The Particle theory of matter 1) All matter is made up of tiny particles 2) All particles in a pure substance are the same; but different than particles in other substances. 3) There is space between the particles. 4) Particles are always moving, the more energy the particle has the faster it moves. 5) Particles in a substance are attracted to each other. 6) The strength of the attraction depends on the type of particle. 2 Lets make a list of fluids in our homes. Viscosity: The term for the resistance to the flow of a liquid. If a fluid has a low viscosity it will flow quickly and be "runny" like water. A high viscosity will be slow and thick, like pancake syrup. Factors that affect Viscosity 1) Temperature and viscosity (Liquids): Since the temperature of a substance affects how much a particle moves around, the higher its temperature the faster it moves and the further away the particles are from each other. So the higher the temperature the lower the viscosity. Ex: warm molasses flows faster than cool molasses. 2) Temperature and viscosity (Gases): Gases have particles that are already very far apart so little friction between them. As you heat a gas you make more and harder collisions between the particles and the gas actually becomes more viscous. 3 3) Concentration and viscosity: Concentration is a term for the amount of substance that is dissolved in a volume of another substance. If you have a larger amount of substance dissolved, there are more particles and more friction between them. So a higher concentration gives you a higher viscosity. Ex: Maple syrup from the tree is very runny, when you boil it down you are removing water. The final product is more concentrated and thicker. 4) Attractive force and viscosity: All particles have an attractive force on each other. This strength is not always the same strength for all substances. So a substance with large attractive forces will have a high viscosity. 5) Particle size and viscosity: The size of the particle also has an effect on the viscosity of a fluid. Larger particles have a harder time moving around than smaller particles do. So a fluid with large particles is more viscous than one with smaller particles. Ex: Margarine that you put on your toast can be soft or hard. The softer margarine has smaller particles of the same type as the hard, so they taste the same but not the same viscosity. Motor Oil... A lesson in viscosity Motor oil needs to be a particular viscosity to do its job. If it is too runny it will not protect the parts of the engine that rub against each other. If it is too thick then it will not get around the engine and coat all the parts that it is supposed to protect. 4 We know that the temperature affects the viscosity of liquids, so motor oil needs to be a certain type to operate at certain temperatures. In Labrador where it is cooler we need a thinner (less viscous) oil because at the lower temperatures when starting the vehicle the oil will still be runny enough to coat the parts. On the island we can still use the thicker oil because it does not get as cold. Oil numbers: 5W-30, 10W-30... The lower the number in front of the W means an oil for colder start temperatures which would be a thinner oil. The second number (30) is the high temperature operating number of the oil for when you are driving around. The "W" is for WINTER and means that the oil is rated for cold weather. Flow Rate This is the speed at which fluids get from one place to another. It is used to compare viscosity, since viscosity is hard to measure directly. Chapter 8 We have already talked about density in Unit 1, where we compared the density of fresh and salt water. Now we will talk about density in greater detail. Density: The amount of mass in an amount of volume for a substance. When we work with mass we use units like grams or kilograms. We say that something has a mass of 500g or something as a mass of 80kg. This is the measure of the amount of matter in the substance. 5 When we work with volume we use units like milliliters or liters or even centimeters cubed. We would say that something takes up a volume of 250ml, or maybe 5L, or maybe 300cm3. It is important to note that 1ml of water is 1cm3 of water and has a mass of exactly 1g. This is how we came up with the unit of the gram. Mass vs Weight Mass and Weight are not the same thing. Mass is a measure of how much matter something has and weight is a measure of how much gravity pulls that mass down. When we ask how much a person weighs we are actually asking for their mass. A person has the same mass anywhere in the universe but has a different weight based on what planet they are on. You have the same mass on the moon as on earth but your weight is much less—about 1/6 of your mass on Earth. (Multiply your Earth weight by 1/6 to get your weight on the Moon). Density Something has a high density if it has a lot of mass in a small volume. Lead is an example of this; even a small piece will feel heavy to you. It has a high amount of mass in a small volume. Something that has a low density like marshmallow will have little mass but take up a lot of space. So how do we calculate density? If mass and volume are important then we need them to calculate density. Since it is an amount of mass for a given volume (mass per volume) we have to divide the mass by the volume. 6 D=m/V Density = Mass / Volume let’s look at an example 5kg / 2L = 2.5kg/L That is to say 2.5 kilograms of mass for every Liter of space taken up by the material. Figuring out volume An easy way to determine the volume of something that is not a geometric figure is to use DISPLACEMENT. This is where you put it in a container filled with water and see how much water it moves out of the way. The amount of water would be the same as the volume of the object. Density change in every day life If you can make the particles of a substance move further apart you can have its mass the same and increase its volume. This will decrease its density. On the other hand if you can make its particles move closer together you can increase its density. Examples: Your car tires need more air in them during the winter because the cold makes the air more dense and your tires are more flat. A hot air balloon uses density too. Heating the air makes it less dense than the air outside the balloon and the balloon rises. Another example is when you leave wood out to dry that you are going to burn. It loses water which makes it lighter and it also makes it better to burn since it has some of its water removed. 7 One of the most interesting examples of density is water. As you cool down water it gets more dense like any liquid but when it freezes into ice which is a solid, it is actually less dense than it was as a liquid. This is why ponds freeze over on the top and your ice cube floats in your glass. Water is most dense at 4 degrees, after that it gets less dense until it gets to 0 where it actually becomes ice. Chapter 9 Force: A force is a push or a pull. Things can exert a force by touching each other like when you pull on a rope, or they can act over a distance like with magnets or gravity. A force can make things move or keep them from moving. Newton: Isaac Newton discovered how gravity works and because gravity is a force, the unit for force is called the Newton (N). Balanced and Unbalanced forces There is often more than one force acting on an object. If all the forces in one direction cancel each other out it is balanced and is either stopped or moves at a constant speed. If the forces do not cancel each other out it will speed up or slow down in that direction. Mass vs Weight revisited Mass we know is the measure of the amount of matter in a substance and is measured in units like Kilograms. Weight however, is the pull of gravity on a piece of matter and can be different on different planets for example. It is measured in Newton’s (N) as we said above. 8 Bouyancy This is the force that water or any other fluid puts on objects as the fluid is moved out of the way. For our rubber ducky example the ducky only pushes a little water out of the way as it sinks into the surface of the water. The volume of water pushed out of the way has the same weight as the weight of the ducky. Anything will float if it can push its weight of water out of the way. If it can’t do this then the buoyancy force will be smaller than the force of gravity pulling down and you will sink. Buoyancy and Density Buoyancy and density are related. If an object is less dense than the fluid that it is in it will always float, no matter what its shape. A piece of wood is a good example of this. However, when the object is denser than the fluid, it has to have a shape that allows it to displace its weight in water and not fill up with the fluid. A steel ship is a good example of this. The second example brings in an idea of Average Density. The steel in the ship is denser than water but the ship also has air in it which is much less dense than water. So the ship on average is less dense than water. Only when water gets in, pushes air out and makes the ship heavier does its density go above that of water and it sinks. This concept of average density is used in nature and by humans. Fish have a swim bladder that they can put air into and out of that changes their density and allows them to swim up and down. Submarines also use this. They have tanks that they can pump water into to sink and pump water out of to rise. Page 341 shows a great diagram of this on the text. 9 Pressure and Fluids Pressure: A force applied over an area. Pressure is measured in Pascals (Pa), however 1Pa is a very small amount so we often use the unit kPa (Kilopascals) which is 1000Pa. How do we get pressure? We need a force to have pressure, if there was no force then there would be no pressure. We also need an area to apply that force. Remember that force is measured in Newtons (N) and area is usually measured in meters squared m2. How do we get a really large pressure? The larger the force the greater the pressure. The smaller the area the greater the pressure, think popping balloons with pins and how it is much easier than popping them with your finger. So, what is our formula for Pressure... how do we get a huge pressure from a force and an area? To get the largest number you need to multiply by the force and then divide by the area since the smaller the area the greater the pressure. Pressure= Force/Area P=F/A We have done calculations earlier in the unit involving density, the calculations using pressure are handled the same as density with the same power triangle but with different symbols in it. 10 Pascal's Law Pascal was a scientist and is who the unit for pressure is named after. His law states that if you apply a force to a fluid that is enclosed, then that force is applied over the entire container. We use this law in our everyday life to our advantage in 2 main ways, with Hydraulic and Pneumatic systems. 1) Hydraulic systems: These use liquids. They have a liquid in a container, apply a force at one point in the system and have it move something in another part of the container. Heavy equipment like a bulldozer and back end loaders are good examples of these systems. 2) Pneumatic systems: These use gases. Just as in above only that we know that gases can be compressed. These are used in things like the shocks of your car, or all the air tools you can think of like an impact gun. Temperature, Pressure and Volume of Gases Temperature has an effect on volume of gases as we already know. As you increase the temperature on a gas you make its volume increase and when you decrease the temperature you make the volume decrease. So increasing temperature will increase the pressure on a gas. On the other hand decreasing the temperature will decrease the pressure. For example a Helium balloon will be a certain size when it has an amount of gas in it. This is because the force pressing out is the same as the pressure pushing in. If you heat the Helium in the balloon the balloon will get bigger because there will be more pressure on the inside and the balloon will grow to spread out the force over a bigger volume. If you put too much pressure on the sides of the balloon it will POP.








