Fossil Fuels - Academic Program Pages at Evergreen
Energy Systems & Climate Change
Tues. 3 Nov.
Ch.5 & 6: Fossil Fuels
Dr. E.J. Zita (& Cheri Lucas Jennings) zita@evergreen.edu
http://academic.evergreen.edu/curricular/energy/0910/home.htm
What’s happening today:
• Questions? Announcements? Check Syllabus
• Finish Ch.5: Fossil Fuel (FF) Energy
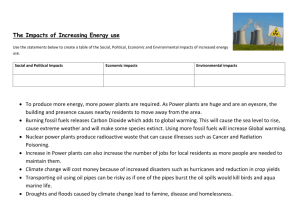
• Start Ch.6: Environmental Impacts of FF
• (No Brief Reports today – they’re Thursday)
• Plato Lecture at 4:00
Homework due this Thursday
Ch.4: Q. 3, 4, 6, 11; Ex. 2, 5, 6, 7, 11, ;
RP: Each team pick one.
Ch.5: Q. 1, 2, 3, 4; Ex. 1, 2, 8, 10;
RP: each team pick one.
We’ve done or started many of these in class together already, and will do more today.
If you want to go faster, please do work ahead on parts or questions we’re not doing in class!
Heat engine
T
T
H
Ch.4 Ex.12: Heat Engine
Ch.4 RP 5: Ocean Warming
If you research the data below, then together we will be able to estimate how much sea level rise is due to the thermal expansion of the ocean (I'll help set this up in class, using your data).
• Estimate the mass in the world's oceans (or look it up).
• How much has ocean temperature increased recently? To what depth? (Check IPCC)
• How much water mass has participated in the bulk of ocean warming?
• What temperature increase do you expect in the next 50-
100 years?
• What is the average ocean temperature now?
Thermal expansion of oceans
Team Awesome (Joyana and Murdoc, 07) found:
Ocean mass m ~ 10 21 kg
Surface temperature has changed 0.5 ºC in the past 35 years
(to what depth? d = _______ m)
Surface area = A = 4 p
R 2 = 3.3 * 10 8 km 2
Current temperature = T
0
= 17 ºC
What temperature increase do you expect in the next 50-100 years?
T = _______
Thermal expansion of water
Change in volume = b
* original volume * change in temperature b
V
0
T where water’s coefficient of volume expansion (at 20°C) is b
= 210 x 10 -6 / °C
Change in volume = area * change in thickness
b
V
0
T
V
0
A d
0
d
Find d
0
_________
Thermal expansion of water
b
V
0
T b
V
0
A d
0
V
0
T
b
( A d
T
0
)
A
b d
0
T
A
d b
T d
0
Thermal expansion of oceans
d b
T d
0 where water’s coefficient of volume expansion (at 20°C) is b
= 210 x 10 -6 / °C, the projected temperature increase in the next century is about
T = 3 °C, and the thickness of the surface layer undergoing most of the heating is d
0
=________________
Sea levels will rise (due to heating alone) about
d =
Ch.5: Fossil Fuel Energy
Origin, composition, and use of fossil fuels
Power plants and Carnot cycle
Reserves and use rates – how long will it last? Exponential functions
Policy issues – carbon tax or cap&trade?
Origin and composition of fossil fuels
The origin of fossil fuels: peat
coal
Plankton, etc.
petroleum
Coal: mostly carbon (with traces of toxins)
Natural gas (mostly methane CH
4
)
Crude oil: 85% C, 15% H, trace S, O, N
Hydrocarbons, e.g. octane: C
8
H
18
CO
2
C
12
text
Fossil-fuel power plant
Q
H
Heat engine
W
Q
L
Heat engine - derivation efficiency
work done
W heat input Q
H
Heat input
work done
heat flow out e
W
Q
H
Q
H
W
Q
L
Q
H
Q
H
Q
L
Q
H
Q
Q Q
H H
L e
Q
L
Q
H
Now we need to showthat
Q
L
T
L
Q T
H H
text
Combined-cycle power plant
e gas
1
T
M
T
H
T
H
T
H
T
M e combined
1
T c
T
H e gas
1
e gas
1
e e
e gas steam
e gas steam
e steam
1
T c
T
M
_________
US oil has peaked
Transitions after peak oil and coal
Energy used time
Total Energy used
area under curve dt
When will the world run out?
Power
energy time
Analogously,
time
______________ if Production
use rate = amount used time and Reserve = amount available
Find the time that a given Reserve will support a given Production: total Production =
Reserve time
time
_____________
Why is this an overestimate?
What if world energy use grows?
Annual rate of energy consumption = N(t) at some time t (units: energy/time)
N
0
= current rate of consumption.
r = rate of growth of energy use (units: 1/yr)
N(t) = N
0 e r t
What is the total energy consumed (C) from now until some future time t?
__________
Integrating exponentials
r t e dt
1 r e r t
t
0 r t e dt
1 r e r t t
0
r
1 e r t e
0
r e r t
1
N
0
e r t
1
r
How long will it take to consume available energy?
C(t) = total energy consumed from now until some future time t. Solve for t.
N
0 r
e r t
1
How long will it take to consume available energy?
C r
N
0
e r t
1
r
e r t
1
N
0
C r e r t
N
0 t ln
C r
N
0
1
ln
1 r ln
C r
N
0
1
r t
How old will you be in 27 years, when proven oil reserves are virtually exhausted? What will you drive? Where will you get plastics?
16.
C = ___________ r = ___________
N
0
= __________
Solve what equation?
Careful with units!
r C
___________
N
0
US-China carbon suicide pact?
You will be able compare these to Romm’s claims (p.201+)
Growth in annual oil imports (1995-2004)
China: 2.8 M bbl/day US: 3.9 M bbl/day
Current demand of global oil exports:
China: 6% US: 25%
China reaches US annual emissions: 2025
China reaches US cumulative emissions: 2050
China: N
0c
=_______________ r c
=____________
US: N
0US
=_______________ r
US
=___________
Sketch the two curves and their future intersection
Policy: Carbon Tax or cap-and-trade?
Tax : Pro: incentive to shift to lower-carbon fuels, such as natural gas
Pro: use tax to fund green energy initiatives
Con: regressive: harder on lower-income
Carbon Cap-and-trade?
Adopted in Europe and elsewhere, for Kyoto
EPA’s cap and trade program effective against acid rain in the US
Pro: market-based incentive, politically viable
Pro: could use income to fund green energy initiatives, dividends to people
Con: you can keep polluting, if you pay
Your thoughts? “Who owns the Sky” has a mathematically symmetric approach that
“reinvents capitalism” … http://www.epa.gov/airmarkets/cap-trade/index.html
EPA cap and trade
http://www.epa.gov/airmarkets/cap-trade/index.html
Action soon can help – remember the
Montreal Protocol
Break, then Ch.6
Ch.6: Environmental impacts of
Fossil Fuels
• CO
2
• Air pollution
• Other environmental impacts
• Policy: Clean Air Act
Ch.6: Q1 & Ex.1
Carbon dioxide CO
2
• non-toxic
• essential for plant life
• potent greenhouse gas (GHG)
28 mpg mpg
6
6
GHG
Air pollution
41 mpg
9/10 (good)
2/10 (bad)
Coal, Natural Gas, & Oil
Recall that Natural gas emits about half the
CO
2 as coal, for the same energy produced and Natural Gas reserves are about the same as world Oil reserves (6000 EJ =
6000 Q, p.128-9)
Air Pollution
• Particulates : smaller = more harmful
• Sulfur emissions : acid rain, cooling aerosols
• Carbon monoxide : toxic, incomplete combustion
• Nitrogen Oxides : NOx from N
2 in air + hot FF
• Photochemical Smog : HC + NOx + O
3
+ …
• Heavy metals : Mercury, lead, …
• Radiation : Coal contains Ur, Th, Ra
Health effects of air pollution
Sulfur emissions
Sulfur + oxygen
SO
2 sulfur dioxide
Oxidation
sulfur trioxide:
2SO
2
+ O
2
2SO
3
Reaction with water vapor
Sulfuric Acid
SO
3
+ H
2
O
H
2
SO
4
Dissolves into sulfate ions:
H
2
SO
4
2H + SO
4
Forms Sulfates: XS0
4
(aerosols can cool climate, briefly)
Carbon monoxide (toxic)
Carbon dioxide from combustion. Ex: methane
CH
4
+ X O
2
A CO
2
+ B H
2
O
Find X, A, B…
What if not all the Carbon forms CO
2
, but some forms CO?
Incomplete combustion
toxic products.
Need Catalytic converter: XCO + YO
2
ZCO
2
Nitrogen oxides
In the presence of hot combustion, nitrogen from the air can form compounds with oxygen
X N
2
+ Y O
2
2 N x
O y
Nitrogen oxides: NOx
Some are less harmful: NO (nitric oxide)
Some are toxic: NO
2
(nitrogen dioxide)
Some are fun: N
2
O
5
(laughing gas)
LA daily smog levels
Why the peaks and variations?
Photochemical smog
Sunlight can release a free O from NOx:
N O
2
+ solar energy
NO + O
The free O readily combines, e.g. into ozone:
O
2
+ O
O
3
Ozone on the ground makes harmful smog (-)
Ozone in the stratosphere protects from UV (+)
Other Environmental Impacts
• Coal Mining
• Oil and natural gas extraction
• Transportation
• Fossil Fuel Processing
• Thermal Pollutione
Mountaintop-removal coal operation
Mercury concentrations in fish
Oil and gas extraction
http://www.britannica.com/ebc/art-95739/North-Sea-oil-drilling-platform-with-natural-gas-flare-off
Oil Transportation
http://www.sitnews.us/0306news/030606/030606_leak_located.html
Canadian Tar Sands
Canadian Tar Sands: http://www.borealbirds.org/tarsands.shtml
Unconventional f.f. resources: oil shale, tar sands, methane hydrates…
Expensive and ecologically damaging to recover and process.
“Double-dirty” (Romm, 182):
1. energy-intensive conversion of tar sands generates 2-4 x GHG per barrel as production of conventional oil
2. Canada uses natural gas to exploit tar sands, instead of selling it as a clean fuel.
Oil spreading into the
Atlantic from a tanker spill off Spanish coast,
2002 (EEC, Fig.6.18)
Fuel Spills
Thermal pollution
http://www.earthscienceworld.org/images/search/results.html?Keyword=Power%20Plants
Policy: Clean Air Act
• Air quality standards
• Emissions standards
Emissions are decreasing, though cars and miles driven are increasing
Looking ahead
Tonight and Wednesday (tomorrow):
• Dig in to Ch.4+5 HW (posted since last week)
Wednesday :
* Office hours 12-1 in 3270 Lab II
• Keep talking with your Research team
• Prepare PIQs for Thursday
Thursday: Class as usual, turn in HW
Next week : Ch.7 – Nuclear Energy
Coal plant tour? Stay tuned…
Centralia Coal Plant
http://www.power-technology.com/projects/centralia/
Break, then Plato Lecture
Energy & Heat
°C = K-273 = 5/9 (°F-32)
Radiation: Light
Power ~ T 4
Carnot cycle: Diesel engine