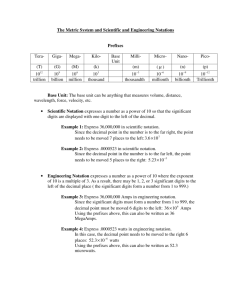
Chemistry & Measurement
advertisement

Get Hand Outs—next 2 projector Mini Quiz #1 9/2/09 • What are four materials that you will need for this class? Wednesday September 2nd, 2009 It is time to take some notes… Chemistry & Measurement Introduction… • Chemists use tools like pipettes to accurately measure liquids. • Some pipettes can be used to accurately measure liquids to tenths, even hundredths of a milliliter. • Careful measurement is vital in scientific investigations. • In Unit 1 you will learn about the nature of science and… • You will also learn how scientists make and record measurements. Introduction… CHEMISTRY Nature of Science Scientific Method Qualitative observations Quantitative Observations Scientific Notation Accuracy & Precision Certainty & Significant Figures Measurement Units & Conversions Big Goals for Learning • To describe science and the scientific method. • To compare qualitative and quantitative observations. • The express numbers in scientific notation • To express accuracy, precision and certainty • To use the rules for significant figures in calculations • To use metric prefixes and conversion factors • To work with units of density and other derived units Objectives • define chemistry • identify qualitative and quantitative observations • list the main steps in the scientific method • describe the nature of science Key Terms • • • • • • • Chemistry— Matter— Mass— Volume— Chemist— Qualitative— Quantitative— Chemistry & the Nature of Science • Science is a system of knowledge about the natural world. • It is also a way of improving and increasing that knowledge. • Latin “scientia” means “to have knowledge”. • Chemistry is one field of science along with field of biology, astronomy, zoology, ecology, etc. Chemistry & the Nature of Science • Chemistry is the study of matter and how it changes. • Matter is anything that has mass and volume. • Mass is a measure of how much matter an object contains. • Volume is the amount of space and object takes up. • Matter is all around you… • The air you breathe, the juice you had for breakfast and you, to name a few. Chemistry & the Nature of Science • Chemists are scientists who study matter and its changes. • They do so with two different types of observations. • Qualitatively, they look and the object; its shape, color, hardness, viscosity, odor, etc. • Quantitatively, they take measurements of the substance to determine its volume, temperature, mass, etc. Chemistry & the Nature of Science • Besides studying the characteristics of a substance, chemists want to know how the substance changes. • Does it react, or combine with, other substances. • How is it that hydrogen gas combines with oxygen gas to make liquid water? • This is the beginning of what chemists do…question their surroundings! Rules of the Lab • Use an indoor voice only to the people in your lab group. Do not wander from table to table. • There is no unauthorized work in the lab. • Use the equipment according to instructions. • Wear protective clothing such as a lab apron. Always wear shoes in the lab. Chemical splash goggles are required when you are working in the lab. • Avoid loose clothing or dangling jewelry; tie back long hair when an open flame is in use. • Always dispose of chemicals in the specified manner. Do not use the drain indiscriminately. • Treat the lab with respect. Mini Quiz #2 9/3/09 1. Name the lab equipment quiz... 2. After the Mini Quiz Get into lab groups and select a leader ASAP...the leader needs to come see Mr. Holt as soon as he/she is elected. Into the Lab we Go! • In your group, you need to elect a leader, reader, artist, labeler. • The leader is to make the lab run smoothly, • know what is going on and keep everyone on task. • If anyone in the group is slacking, it is the leader’s job to get them back on task. • Ultimately if the team fails, the leader fails. • Only the leader may speak with Mr. Holt. Into the Lab we Go! • The reader’s job is to read the question to the group. • They are also supposed to read the index cards throughout the room to the group. • The reader may not speak with Mr. Holt at any time during the lab Into the Lab we Go! • The artist’s job is to design the blueprint for the poster board. • They may ask the group for ideas but they are not required to. • The leader may force the artist to work with the group. • The artist may not speak with Mr. Holt at any time during the lab • As you travel from station to station, it is the artist’s job to make sure everyone understands each index card. Into the Lab we Go! • The labeler’s job is to come up with the “balloons” of descriptions on the poster board. • They may ask the group for ideas but they are not required to. • The leader may force the labeler to work with the group. • The labeler may not speak with Mr. Holt at any time during the lab • The labeler must design a name sheet for the group with a team name, team members and a logo. • The labeler must carry this around with the team. Into the Lab we Go! • The goal of the lab group is to divide up tasks, but work as a team. • Every member of the group must answer the questions of the handout and they must be able to answer any questions Holt may have about the lab or lab equipment. • The only time a group member other than the leader can speak to Mr. Holt is if he asks you a question. More Key Terms • • • • • • • Scientific method— Hypothesis— Variable— Independent variable— Dependent variable— Theory— Technology— The Scientific Method • Science is a system of knowledge. • It is based on observations about the natural world. But it is more than that. • It is also a process of learning about the natural world. • Scientists use a process called the scientific method to answer questions or solve problems. • They try to explain why matter behaves like it does. The Scientific Method • The scientific method is a way of improving and increasing existing knowledge based on existing facts and ideas, new observations and reason or logic. The Scientific Method • The process can vary quite a bit but it often involves the following steps: • Observe the natural world. • Ask a question or state a problem based on observations. • State a hypothesis based on facts—a hypothesis is a possible explanation based on facts and reason. It predicts what might happen. The Scientific Method • Test the hypothesis by designing and performing experiments. • The qualitative and quantitative observations made during experiments are called data or results. • Analyze the results. • This means organizing the data, looking for patterns and making sense of the data. The Scientific Method • Share the results and conclusions with others stating whether the experimental data supported the hypothesis or not. • If not, what things might you add or change in the experiment or hypothesis to bring us closer to truth. • Scientists share their findings in published journals so that scientist all over the world can read and reproduce their experiments comparing results and checking for errors. Experimenting • In many experiments, certain conditions or characteristics of matter are measured or controlled. • These are variables. • Variables may change during an experiment or they may be held constant. • Volume, temperature, type of substance and time are all variables. Mini Quiz #3 (9/8/09) • Get hand out of HW problems... • Then for our Mini...name the piece of glassware...from this site: • http://www.sciencegeek.net/Chemistry /taters/labequipment.htm • Copy down my blog site...there will be info placed here throughout the year. • http://blogs.muskegonisd.org/mholt Experimenting • Some experiments are designed to see how one variable changes when another variable is changed. • The variable that is changed by the experimenter is the independent variable. • The other variable is the dependent variable—it responds to the independent variable. Example Under lamp for 1 hour Under lamp for 2 hours Under lamp for 3 hours After the time goes by the chemist measures the volume of water that remains after evaporation… Independent variable = time, dependent variable = volume of water that evaporates due to the changing time under the heat lamps Which is Which? • A chemistry student is trying to determine what factor will make 2.0g of salt crystals dissolve the quickest. He tells you that his teacher gave him a poor grade on his set up. He tells you his set up and it is your job to help him fix it. This is what he did... • He took 2.0g of salt crystals and put them into 100mL of 30ºC water and let it dissolve. He recorded the time that it took to dissolve all the crystals. Then he took 2.0g of salt crystals and put them into 500mL of 80ºC water and stirred the solution. • What would you suggest...write up your plan. Which is Which? • Possible set up... • Get three beakers of 200mL of distilled water— two beakers at 30ºC and the 3rd at 80ºC. • Into the first beaker dump 2.0g of salt crystals in and record the time to dissolve. This is the control and all the other tests should be based around this one. • To the 2nd beaker...add the 2.0g of salt and stir it with a spoon until all the salt has dissolved. Record the time. • Then to the 3rd beaker...add the 2.0g of salt and do not stir—let the salt dissolve and record the time. Which is Which? • The independent variable is the variable that the chemist changes. In test #2, what is the independent variable? • The dependent variable is the variable that changes because the chemist change something from the control. What is the dependent variable in the 2nd test? • What is the independent variable in the 3rd test? • What is the dependent variable in the 3rd test? Analyzing • One way to analyze the results of an experiment is to make a graph. • The relationship between the two variables can be seen on the graph. • The independent variable is plotted on the x-axis and the dependent variable on the y-axis. Other Stuff to Know • Experiments cannot prove that a hypothesis is correct. • However, scientists perform the experiments many times. • Each time new results support the hypothesis, the hypothesis become more likely. • If the results of many experiments support the hypothesis, scientists may call this hypothesis a theory. • A theory is a well-tested hypothesis that is widely accepted. Other Stuff to Know • An example is the atomic theory of matter. • It explains matter in terms of atoms and the tiny particles that make up atoms: protons, neutrons and electrons. • Many experiments have supported the idea that matter is made of these tiny particles. The Science of Nature • People of all cultures have been observing and studying the world for many centuries. • Because of this, the body of scientific knowledge continues to grow and change. • A new discovery may change an existing theory. • New results may give meaning to old observations. • New tools may measure or show something for the first time. • As the process of science answers one question, it leads to many new questions. The Science of Nature • When scientific knowledge is used in a practical way to improve lives, the result is called technology. • For example, if a chemist comes up with a drug that slows a human disease, that development is called technology. • Scientists generally do not focus on ways to apply what they learn. • Instead, engineers apply scientific knowledge and create technology. Mini Quiz #4 (09/09/09) • List and identify all of the qualitative (QL) and quantitative (QT) observations… • On the 3rd day of September I was working in the lab with 5.0g of a white, crystalline solid. My teacher asked me to place it in 250mL of a clear liquid with no odor. I measured and recorded the mass of the liquid to be 250g. When I placed the solid in the liquid I noticed that the solid dissolved. When I placed the beaker on the scale it now read 255.0 g. Objectives • Write a number in scientific notation • Convert a number in scientific notation to standard notation • Give examples of accurate measurements and precise measurements • Know how the quality of a measuring tool affects certainty • Identify the significant figures in a measured value Key Terms • • • • • Scientific notation— Superscript— Accuracy— Precision— Significant figure— Scientific Notation • Scientists often have to measure very large amounts or very small amounts. • To make it easier to work with, they write numbers in scientific notation. • Scientific notation is a shortcut that uses powers of 10. • Powers of 10 are written as 10x, where x is a number called and exponent. • The exponent is shown as a superscript, a number written just above the writing line. • The exponent tells how many times 10 is multiplied by itself. (103 = 10 x 10 x 10 = 1000) Scientific Notation • There is an easy way to express a number in scientific notation. • Just move the decimal point in the given number to create a number that is between 1 and 10. • For example 3,470,000 the decimal is moved from behind the last zero to be in between the 3 and the 3 to get 3.47. Scientific Notation • Another…0.00000567 the decimal is moved to go in between the 5 and the 6 to make 5.67. • Next count the number of places you moved the decimal point and that is your power of 10… • Example 1, the decimal was moved 6 places and example 2, the decimal was moved 6 placed. • When the number greater than 1, the exponent is positive, when it is less than 1 the exponent is negative. So… • Example 1 = 3.47 x 106 • Example 2 = 5.67 x 10-6 Example • Write the number 765,000,000,000 in scientific notation. • 7.65 • Moved the decimal 11 places • Number is greater than 1 so… • 7.65 x 1011 • Try 0.000000349 • 3.49 x 10-7 • Write 3.2 x 10-4 in standard notation • 0.00032 Objectives • Give examples of accurate measurements & precise measurements • Know how the quality of a measuring tool affects certainty • Identify the significant figures in a measured value Key Terms • Accuracy— • Precision— • Significant figure— Accuracy, Precision and Certainty • Are some measurements better than others? • Do measuring tools affect measurement? • Does it matter how measurements are written down? Accuracy, Precision and Certainty • In science it is important to make measurements that are both accurate and precise. • Accuracy is how close a measurement is to the correct or accepted value. • Precision is how close a measurement is to other measurements of the same thing. Accuracy, Precision and Certainty • Dart board analogy of accuracy and precision. Accuracy, Precision and Certainty • Scientists work carefully to obtain accurate and precise measurements. • Scientists also need to know how certain their measurements are. • After measuring something, scientists record the measurement as a number. • In any number that represents a measurement, there are digits that are certain and one digit that is uncertain. • The last digit on the right in the number is an estimate, or best guess. • This digit is the uncertain one. Accuracy, Precision and Certainty • Imagine stepping a scale and it reads 125 pounds. • The 5 (furthest number to the right) is an uncertain measurement. • If you used a better quality scale it might read 124.8 pounds. • In this case the uncertain digit would be the 8. • The value of 124.8 pounds has greater certainty than 125 pounds so the better quality scale is gives the more certain measurement. • The certainty of measurement depends on the tool that is used. Accuracy, Precision and Certainty • Which ruler will give a more certain measurement? 1 cm 1 cm 2 cm 2 cm 3 cm 3 cm Significant Figures • It is important that scientists correctly record the certainty of their measurements. • For any measurement value, scientists only record all of the certain digits plus one uncertain, or estimated, digit. • The uncertain digit is the last one on the right. • Together there are the meaningful digits or significant digits. Significant Figures 124.8 pounds 3 certain digits 1 uncertain digit 4 significant digits total Example 1: A scientist records a measurement of 6.2345 meters. All of the digits are significant. Which digits are uncertain? • Read: The number is 6.2345 is a measured value that has 5 significant digits. • Plan: The significant digits in a measured value always include one uncertain digit at the far right. • Solve: In the number 6.2345m the 6, 2, 3, 4 are certain. The 5 is uncertain. • Reflect: every measured value contains one uncertain digit. The rest are certain. • Practice: A scientist records a measurement of 57 seconds. Both digits are significant. Which one is uncertain? • Answer: The 7. Significant Figures • Scientists use the following set of rules for counting the significant digits in a measurement: • Rule 1—Nonzero digits are significant. • Rule 2—Final zeros to the right of the decimal point are significant. • Rule 3—Zeros between two significant digits are significant. • Rule 4—Zeros used for spacing the decimal point are not significant. • Rule 5—For numbers in scientific notation, all of the digits before “x10y” are significant. Significant Figures • • • • • • • • • • • Get out the boards, how many sig figs?… 13.53 4.6025 200035 0.0000300 2.0000300 0.002 4.44 x 103 10.00 10 10. Mini Quiz #5 (9/10/09) Our First Quest will be on Tuesday! 1. Imagine that you are working on an experiment to determine how reliable your scale is. Sounds fun, huh? You place a 1kg mass on the scale and it reads 995g. Is the scale accurate, precise, both, neither or are you unsure and need to carry out further testing? If unsure, explain why? 2. How many significant figures in the following… a) 21.02 b) 21.00 c) 0.020 d) 500 e) 5.00 x 102 Objectives • Give the correct number of significant figures when multiplying and dividing measurements • Know the difference between a measurement, a defined number, and a counting number • Give the correct number of significant figures when adding and subtracting measurements Solving Problems with Significant Figures • Many chemistry problems involve measurements. • Measured values are often multiplied of divided. • Significant figures are important when solving these problems. Multiplying and Dividing with Significant Figures • Suppose you measure a room and find that it is 22 feet long and 9 feet wide. • The length value, 22, has 2 significant figures. • The width value, 9, has 1 significant figure. • The area of the room is length x width: • A = 22 feet x 9 feet = 198 square feet rounded to 200 square feet Multiplying and Dividing with Significant Figures • Why was the number rounded to 1 significant figure? • When multiplying or dividing measurements, the answer must have the same number of significant figures as the measurement with the fewest significant figures. • Often the answer from a calculator has more figures than allowed by this rule so the value must be rounded. Multiplying and Dividing with Significant Figures • • • • • • • • • • • Examples… 2.86ft x 1.824ft = 5.21664ft2 5.22ft2 21mi / 8h = 2.625 mi/h 3 mi/h 98.0in x 1.22in = 119.56 in2 120. in2 or… 1.20 x 102 in2 Multiplying and Dividing with Significant Figures • Sometimes a number in a problem is not a measurement. • It might be a defined number. • A defined number is part of a definition and is not measured. • For example, suppose you measure something that is 725cm long and you want this measurement in meters. Multiplying and Dividing with Significant Figures • You find that 100cm = 1m. • You divide 725cm by 100 to get 7.25m • Do you write this as 7m because 100 has only one significant figure? • No, the number 100 is part of a definition and defined numbers do not limit the significant figures in an answer. Multiplying and Dividing with Significant Figures • In another case, a number in a problem might be a counting number.’ • Say you have a 28-inch sub sandwich and you want to split it into 5 equal pieces. • The number 5 is a counting number, not a measurement. • Counting numbers, like defined numbers, do not limit the number of significant figures so, report the answer to 2 significant figures, not 1. Adding and Subtracting with Significant Figures • When adding or subtracting measurements the amount of significant figures reported in your answer must be the same number of decimal places as the least certain measurement (fewest decimal places). • 4.271g + 2g + 10.0g = 16.271g = 16g Objectives • Name common base units • Identify metric prefixes and know how they change a base unit • Use conversion factors Key Terms • • • • • • • • Unit— Meter— Gram— Liter— Kelvin— Degree Celsius— Unit Conversion— Conversion Factor— Measurement Units and Unit Conversions • An important part of the scientific method is sharing results with other scientists. • Results usually include measured values that have units. • A unit is a standard amount used for measuring • There is usually more than one unit for measuring something. • Meters, yards and feet are all used for measuring length. • Each of these units represents a different standard distance. Base Units • In 1960, scientists around the world agreed on one system of measurement called the International System of Measurement or (SI). • This system uses: – – – – – meter (m) for length gram (g) for mass Liter (L) volume Kelvin (K) for temperature (ºC is also used) second (s) for time (min or hr are also used) Metric Prefixes • The base units just viewed are sometimes too big or too small depending on what is being measured. • You wouldn’t want to measure the area of a book in meters or the mass of an elephant in grams so prefixes are used. • You must know: • micro- 0.000001 (1 x 10-6) • milli- 0.001 (1 x 10-3) • centi- 0.01 (1 x 10-2) • deci- 0.1 (1 x 10-1) • kilo- 1000 (1 x 103) Unit Conversions (Factor Labeling) • • • • • • All measurements consist of a number and a unit. Using the correct unit is important. Without a unit, a measurement has no meaning. Using a wrong unit can also cause trouble. There is a big difference between 8s and 8hr. Unit conversion is the process of changing a measurement form one unit to another. • Some conversion factors are 1 pound = 454g, 1 gal = 3.8L, 1km = 0.62mile, 2.54cm = 1 inch • Examples of factor labeling (unit conversion)… Examples… • • • • • • How many grams are there in 2.2 tons? There are 2000lb in 1 ton. There are 454g in 1 lb. How fast in m/s is 55mi/h? There is 1609m in 1 mile. There are 3600s in 1 h? Chemistry To Do… 1. Get your mini quiz books. 2. Turn in your Poster on the desk in the back of the room—you may not work on your Poster now—it is due now. 3. Sit with a smile and get ready for some mini-quiz fun! Mini Quiz #6 (9/11/09) 1. How many significant figures are in the following numbers... • 20.023cm • 13.0cm • 0.0210cm 2. Multiply the 3 measurements above and record with proper significant figures. 3. Add the 3 measurements above and record with the proper significant figures. 4. Use factor label to convert 1.10 miles to mm. (5280ft = 1 mile, 12in = 1ft, 2.54cm = 1in, 10mm = 1cm) Mini Quiz #6 (9/11/09) 1. How many significant figures are in the following numbers... • 20.023 • Five • 13.0 • Three • 0.0210 • Three 2. Multiply the 3 measurements above and record with proper significant figures. • = 5.466279cm3 • = 5.47cm3 Mini Quiz #6 (9/11/09) 3. Add the 3 measurements above and record with the proper significant figures. • 33.044cm • 33.0cm 4. Use factor label to convert 1.10 miles to mm. (5280ft = 1 mile, 12in = 1ft, 2.54cm = 1in, 10mm = 1cm) Practice Time • http://www.sciencegeek.net/APchemistry/ APtaters/chap02counting.htm Objectives • Give 2 examples of derived units • Calculate the density of an object • Calculate the mass (or volume) of an object, given its density and volume (mass). Key Terms • Derived unit— • Density (D)— Derived Units • Suppose you measure the length and width of a room in meters. • You could multiply these two measurements to find the area of the room. • Because length and width are both measure in meters, the unit for area is square meters (m2). Derived Units • If you measured the room’s height, you could determine the volume (length x width x height) in cubic meters (m3). • Both m2 and m3 are called derived units—measurements units created by multiplying or dividing other units. • Density is a derived unit where mass is divided by volume. D = m / V Derived Units • Calculate the density of a substance with a mass of 24.3g and a volume of 32.9mL. Use the correct unit and the correct number of significant figures in your answer. • D = 24.3g / 32.9mL = 0.7386018g/mL = 0.739g/mL Derived Units • What is the volume of an object with a density of 1.25g/mL and a mass of 281g? • D=m/V • DV = m • V=m/D • V = 281g / 1.25g/mL • V = 224.8mL = 225 mL • Fun fact: the density of pure water is 1 g/mL. • Another Fun Fact is that 1mL = 1cm3 (Know this) Review • Mass divided by volume is called _______. • The amount of space an object takes up is its ________. • After a hypothesis has been tested many times, it may become a(n)__________. • In a measured value, a zero that is used only to space the decimal point is not ___________. • A unit of mass is the _______. • The __________ of a measurement is how close the measurement is to the true or accepted value. Review • The ____________________ is used by scientists to answer a question or solve a problem. • Large and small numbers can be written more easily using ________________. • Area is an example of a quantity requiring a(n) ____________. • A unit for volume is the __________. • A(n) _________ is a possible explanation based on facts and reason. • The amount of matter in an object is its ________. Review • The substance began to dissolve is an example of a _____________ description. • The number 3,337 has ____ significant figures. • The number 0.00529 is written as ___________ in scientific notation. • A beaker is 152g. A student measures the mass of this beaker three times and gets these results: 137g, 136g and 137g. The measurements are ________________________________. (according to accuracy and precision) Review • Multiply these measurements: 0.4330cm x 209,000cm. • Convert 75.3cm to mm. • Convert 0.186kg to mg. • A substance has a mass of 257g and a volume of 352mL. Find its density in grams per milliliter. • A substance has a density of 0.876g/mL and a volume of 25.6mL. Find its mass in grams. Review • Give an example of a number that has one zero that is significant and one zero that is not. • Show the steps involved in converting 10L into mL using factor label. • A substance has a density of 1.52g/mL and a mass of 220g. What is its volume in milliliters. What do you know? • In which of the following pairings of metric system prefix and power of ten is the pairing incorrect? a) b) c) d) kilo- and 10-3 micro- and 10-6 deci- and 10-1 mega- and 106 What do you know? • Which of the following statements about the “significance” of zeros in recorded measurements is incorrect? a) b) c) d) leading zeros are never significant confined zeros are always significant trailing zeros are never significant trailing zeros may or may not be significant What do you know? • The estimated digit in the measurement 65,430 seconds is a) b) c) d) the the the the zero three four five What do you know? • When rounded to three significant figures, the number 43,267 becomes a) b) c) d) 432 433 43,200 43,300 What do you know? • The uncertainty associated with the measurement 0.3030 lies in the a) b) c) d) Tenths place (0.1) Hundredths place (0.01) Thousandths place (0.001) Ten-thousandths place (0.0001) What do you know? • The number 273.00, when expressed in scientific notation becomes a) b) c) d) 2.73 x 10-2 2.7300 x 10-2 2.73 x 102 2.7300 x 102 What do you know? • The calculator answer obtained by multiplying the measurements 53.534 and 5.00 is 267.67. This answer a) b) c) d) is correct as written should be rounded to 267.7 should be rounded to 268 should be rounded to 270 What do you know? • The calculator answer obtained by adding the measurements 8.1, 2.19 and 3.123 is 13.413. This answer a) is correct as written b) should be rounded to two significant figures c) should be rounded to 13.41 d) should be rounded to 13.4 What do you know? • What is the volume, in milliliters, of 50.0g of a liquid if its density is 1.20 g/mL? a) b) c) d) 32.1 mL 41.7 mL 60.0 mL 75.0 mL What do you know? • Which of the following statements concerning the three major temperature scales is incorrect? a) Kelvin scale temperatures can never have negative values. b) A Celsius degree and a Kelvin are equal in size. c) The addition of 273 to a Fahrenheit scale reading will convert it to a Kelvin scale reading. d) The freezing point of water has a lower numerical value on the Celsius scale than on the Fahrenheit scale. For Today in Chemistry… • Today is review for our first quest which is tomorrow. • You do not need your mini quiz books. • Please get in the following arrangement now… Only the captain/reader can see the board. Writer Captain/ Reader Only the captain can speak, unless Mr. Holt says. After each problem, rotate 1 seat in a clockwise fashion…Captain to Solver, Solver to Writer, Writer to Helper and Helper to Captain. Practice for Quest 1. A piece of turquoise is a blue-green solid, has a density of 2.65g/cm3, a mass of 2.5g, and a length of 2.6cm. Which of these observations are quantitative and which are qualitative? 2. A marathon runner covers a distance of 40.0km. What is that distance in meters? In miles? 1 mile = 1609m. 1000m = 1km Practice for Quest 3. When you heat popcorn, it pops because it loses water explosively. Assume a kernel of corn, with a mass of 0.125g, has a mass of only 0.106g after popping. What percent of its mass did the kernel lose on popping? 4. Popcorn is sold by the pound in the U.S. Using 0.125g as the average mass of a popcorn kernel, how many kernels are in a pound of popcorn? (1 lb = 453.6g) Practice for Quest 5. A particular paint has a density of 0.914g/cm3. You need to cover a wall that is 7.6m long and 2.74m high with a paint layer 0.133mm thick. (a) What volume of paint (in L) is required? (b) Oh yeah, what is the mass (in g) of the paint layer? Practice for Quest 6. You and your lab partner are asked to determine the density of an aluminum bar. The mass is known accurately (to four significant figures). You use a simple metric ruler to measure its dimensions, and after calculating the volume, you determine the density given in the table (Method A). Your partner uses a precision micrometer to measure the dimensions and then calculates the density (Method B). Method A (g/cm3) 2.2 2.3 Method B (g/cm3) 2.703 2.701 2.7 2.4 2.705 5.811 If the accepted value of density is 2.702 g/cm3, what is the average accuracy of Method A and B? Which method is the most precise? Practice for Quest 7. What is the sum AND the product 10.26cm and 0.063cm? 8. What is the result of the following calculation? (7.779) x (0.0546)(16.0000) (55.85) Practice for Quest 9. A mineral oil has a density of 0.875 g/cm3. Suppose you spread 0.75g of this oil over the surface of water in a large dish with a diameter of 21.6cm. How thick is the oil layer? Express the thickness in centimeters. Always use the correct significant figures. The area of a circle is, of course, A = πr2. Practice for Quest 10. Liquid nitrogen boils at 77K. What is this temperature in Celsius degrees, when K = ºC + 273? 11. The density of air at 0ºC is 1.293 x 10-3 g/cm3. What is the density of air at this temperature in grams per liter? 12. The density of mercury at 0ºC is 13.595 g/mL, at 10ºC it is 13.570 g/mL and at 20ºC it is 13.546 g/mL. Estimate the density of mercury at 30ºC. Practice for Quest 13. The volume of a cube is 512 cm3. What is the volume of this cube in cubic meters? 100 cm = 1m 14. Also know • the difference between matter, mass & volume • all the glassware (www.sciencegeek.net for review) • the steps to the scientific method • control, independent and dependent variables • scientific notation