Investigation 11A Stoichiometry 2
advertisement

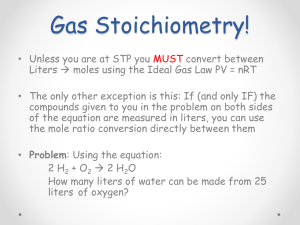
Investigation 11A Stoichiometry If your doctor did not tell you how much of a medication you need to take, you could take too little and the medicine would not work. But if you take too much, it could cause severe consequences. How do you know how much of a chemical is necessary for a reaction to occur? 2 Investigation 11A: Stoichiometry Part 1: The reaction 1. Put an empty cup on the balance and zero it. Measure 10.0 g of baking soda in the cup. Record the measured mass in Table 1. 2. Put another empty cup on the balance and zero it. Measure 30.0 g of vinegar in the cup. Record the measured mass in Table 1. 3. Add the masses of vinegar and baking soda. Record the sum in Table 1 (row 3). 3 Investigation 11A: Stoichiometry Part 1: The reaction 4. Slowly pour the vinegar into the baking soda. Pouring it too fast might cause the bubbling to overflow! Wait until the bubbling has stopped and record the mass. 4 Investigation 11A: Stoichiometry Part 2: What happened? a. What evidence did you observe that indicated a chemical reaction was taking place? b. Explain the difference in the total mass before and after mixing. c. Do you see any baking soda that has not reacted? 5 Investigation 11A: Stoichiometry Part 3: Understanding the reaction Baking soda (sodium bicarbonate) Acetic acid (acid in vinegar) a. The balanced reaction is written on the first line of the chart above. Calculate the formula mass of each of the products and reactants. 6 Investigation 11A: Stoichiometry Part 3: Understanding the reaction 84.00 Baking soda (sodium bicarbonate) 60.05 82.03 44.00 18.01 Acetic acid (acid in vinegar) b. One of the products is a gas. Which one? Will this product’s mass contribute to the mass as measured on the balance after the reaction has finished? Why or why not? c. On the second line, write down the mass of the baking soda and vinegar you added. 7 Investigation 11A: Stoichiometry Part 3: Understanding the reaction 84.00 60.05 82.03 44.00 18.01 d. Vinegar is 5% acetic acid (HC2H3O2) by mass. Multiply by 0.05 to get the actual mass of acetic acid. e. Use the formula masses to calculate the number of moles of NaHCO3 and HC2H3O2. 8 Investigation 11A: Stoichiometry Part 3: Understanding the reaction 84.00 60.05 82.03 44.00 18.01 f. How many moles of CO2 were produced? g. Use the formula mass to calculate the mass of CO2 produced. 9 Investigation 11A: Stoichiometry Part 4: Stop and think a. How many grams of CO2 were produced in the reaction? b. How does the answer to Part a explain the mass measurement you found at the end of the experiment? c. Did all the baking soda react? How do you know? d. Did all the acetic acid react? How do you know? e. What was the limiting reactant in this experiment? How do you know? 10 Investigation 11A: Stoichiometry Part 5: An efficient reaction To make an efficient reaction, there should not be any reactants left over. 84.00 60.05 Steps a–d Use this chart to calculate how many grams of vinegar need to be added to react with all the baking soda. 11 Investigation 11A: Stoichiometry Part 6: Test your hypothesis 1. Measure out 10.0 g of baking soda in one cup. 2. Measure out the required amount of vinegar (from your calculations) in the other cup. 3. Write down the total mass before you mix the reactants. 12 Investigation 11A: Stoichiometry Part 6: Test your hypothesis • Calculate the mass of CO2 produced. • How does this number match your experiment? 84.00 13 60.05 82.03 44.00 18.01 Investigation 11A: Stoichiometry Part 7: Using stoichiometry Steps a–e Using stoichiometry, calculate how much baking soda and vinegar you would need to make 10.0 g of CO2. 84.00 14 60.05 82.03 44.00 18.01 Investigation 11A: Stoichiometry Part 8: Applying the principle Perfect combustion of octane: If the fuel is burned perfectly, the reaction that occurs in a gasoline engine would only produce carbon dioxide and water. 15 Investigation 11A: Stoichiometry Part 8: Applying the principle Perfect combustion of octane: a. Calculate the formula mass for octane. b. Suppose a car uses 25 gallons of gasoline in a week. The density of gasoline is about 2,900 g/gallon. Calculate the mass in grams of 25 gallons of gasoline. 16 Investigation 11A: Stoichiometry Part 8: Applying the principle Perfect combustion of octane: c. Assume gasoline is pure octane. How many moles does this quantity represent? d. How many moles of CO2 are created for every 2 moles of gasoline burned? e. Calculate the mass of CO2 released from the perfect combustion of 25 gallons of gasoline. 17 Investigation 11A: Stoichiometry