L6 Penny Lab, Informal
advertisement

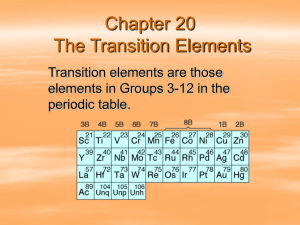
Three Penny Lab Informal Chemistry – Lab #6 Performance date: Friday, 3/27/2015 Due date: Monday, 3/30/2015 (2 pts. extra credit if received today) Three Penny Lab Chemistry – Lab #9 Physical Science – Lab #8 • Goggles and apron a must. Wipe up spills immediately. • The solution is mixed up for you already. If your two pennies don’t get “coated” in a reasonable amount of time, let me know. • Leave crucible on the hot plate and DO NOT let it boil. (Obnoxious fumes will be produced if boiled.) Safety and Cleanup Goggles, Aprons, Tie back long hair, DON’T let solution boil. Wipe up spills immediately. Avoid: heat and chemical burns Use metal tools (forceps) provided Cleanup: Wipe up counters with sponge Throw all used paper towels away Leave evaporating dish on hot plate Turn down hot plate ***Rinse off forceps, glass rod, and beaker and leave at your station. Informal Report • One cover sheet per group • Per Person: (stapled with 3x5 card on top) – One 3x5 card with name, period and 3 pennies – Copper Silver Gold • Attach: Completed questions #1- #15 on hand out. IX Discussion Section 1. What is an alloy? 2. Discuss the means by which the copper and zinc atoms form an alloy. 3. What is unique about a metallic bond that makes it completely different from all other bond types? 4. How many valence electrons does copper have? Zinc? 5. Why is the solution of zinc and sodium hydroxide heated before the copper pennies are added? 6. What are the chief characteristics of metals? 7. What is solubility? What factors effect solubility? 8. What are the four characteristics of a metallic bond? 9. What is the relative strength of the metallic bond between zinc and copper (both transition metals) in the formation of Bronze. 10. What special characteristics of zinc and copper make it readily bond together to form brass? 11. What are the sizes of the atomic radii and ionic radii of the metals involved? 12.What are the ionization energies of the metals involved in the bond? 13.What are the electron affinities of the metals involved in the bond? 14. What are the electronegativities of the metals involved in the bond? 15. What do we know about the activity of these two metals involved in the bond?