Biochemistry 1 (BASIC-106)
advertisement

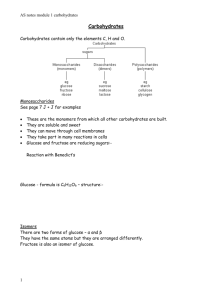
•Dr. Hewida Fadel •Dr. Tarek El Sewedy Intended Learning Outcomes By the end of this lecture, students will learn: 1. The basic structure and classes of carbohydrates. 2. Biological function of carbohydrates Lecture content What are carbohydrates? Classification of carbohydrates. Classification of monosaccharides. Reducing & non reducing sugars. Classification of polysaccharides. Biological function of carbohydrates Biomolecules of life 1. Carbohydrates 2. Proteins. 3. Lipids 4. Nucleic acids What are carbohydrates? In biochemistry, carbohydrates is a synonymous of saccharide. The word saccharide comes from the Greek word sákkharon, meaning “sugar". The term carbohydrate often means any food that is particularly rich in the comples carbohydrate starch (such as cereals, bread, and pasta) or simple carbohydrates, such as sugar (found in candy, jams, and desserts). Carbo-hydrate is composed of carbon and water (C.H2O)n. For every carbon there is 1 water molecule or 2 hydrogen atoms and 1 oxygen atom (with some exceptions). Classification of carbohydrates There are a variety of classification schemes. The most common classification scheme divides the carbohydrates into groups according to the number of individual simple sugar units. Monosaccharides contain a single unit disaccharides contain two sugar units (2 monosaccharides) polysaccharides contain many sugar units as in polymers Monosaccharides can be further classified by the number of carbons present. Six carbons = Hexose (ex. Glucose, Galactose,fructose) Five carbons = Pentose (ex. Ribose) Four = Tetrose (Erythose ) Three = Triose (Glyceraldehyde) Modified monosaccharides phosphorylated sugars are one example of modified monosaccharides. An important phosphorylated sugar is glucose 6-phosphate. glucose 6-phosphate provides energy in certain metabolic pathways, and it can be converted and stored as glycogen when blood glucose levels are high. If blood glucose levels are low, glucose 6-phosphate can be converted back into glucose to enter the bloodstream again. glucose 6-phosphate possesses a negative charge. This prevents the molecule from leaving the lipid-bilayer membranes. This allows the cell to easily uses the modified sugar to provide energy, or convert it to glycogen as storage. Classification according to Functional Groups • Aldoses: Are sugars containing an aldehyde functional group on carbon #1 (anomeric carbon)- Monosaccharides in this group are glucose, galactose, ribose, and glyceraldehyde. • Ketoses: Sugars containing the ketone group on carbon # 2 (anomeric carbon)The major sugar in this group is fructose. Classification according to reducing capability Reducing capability is defined by the presence of free aldehyde or ketone group. A. Reducing: Contain free groups (contain one free anomeric carbon atom), glucose, maltose, lactose and fructose. • All Monosaccharides are reducing sugars. Two of three disaccharides maltose and lactose, have the free groups needed to act as reducing agents. B. Non-reducing: Contain no free groups. Sucrose and polysaccharides are non reducing. (sucrose's anomeric carbon is not "free" since this carbon is used to link fructose and glucose together. Reducing Disaccharides Lactose Maltose Non reducing disaccharide Classification of polysaccharides Homo-polysaccharides (all the same type) Hetero-polysaccharides (mixtures of monomer types) Complex carbohydrates (joined to non-carbohydrate molecules) fructose glucose galactosea monosaccharaides… joined together to make disaccharides. sucrose (fructose-glucose) maltose (glucose-glucose) Disaccharides lactose (glucosegalactose) Glucose Starch (unbranched) Starch (branched) Glycogen Polysaccharides Cellulose Carbohydrate Function • Sources of Immediate energy (ATP) as produced by glucose catabolism(Glycolysis and Krebs cycle). • Source of stored energy (Glycogen stored in liver). • Intermediates in the biosynthesis of other basic biomolecules (fats and proteins). • Associated with other molecules such as vitamins and antibiotics. Cont, Carbohydrate function • Structural function: ex. Ribose, deoxyribose and cellulose and starch in plants. • Involved in many cell functions such cell-cell recognition and protein folding. Digestion of carbohydrates a) α-amylase (in saliva) randomly hydrolyzes all the glycosidic bonds of starch. • By the time the chewed food reaches the stomach, where acidity deactivates the amylase, the average chain length of starch has gone from several thousand to fewer than 8 glucose units. b) Digestion continues in the small intestines with pancreatic amylase. This degrades the starch to maltose . c) Further digestion occurs down the digestive tract in the small intestine. The resulting monosaccharides are absorbed by the small intestine and transported into the blood stream • In living organisms, most carbohydrates are found bound to other compounds rather than as simple sugars Glycoproteins (hormones, antibodies) Glycolipids Nucleic acid. Monosaccharaides • Monosaccharaides are also known as “simple sugars” • They are classified by: 1. Number of carbons. 2. Whether they are aldoses or ketoses. • D-glyceraldehyde is the simplest of the aldoses. • Glucose is the most widely known Glucose Glucose is a monosaccharide with formula C6H12O6 It is absorbed directly into the bloodstream during digestion. Bread, rice, pasta are rich in glucose. Glucose is a common medical analyte measured in blood samples. The insulin reaction, regulate the concentration of glucose in the blood. A high fasting blood sugar level is an indication of diabetic conditions Glucose is used as an energy source in most organisms aerobic or anaerobic respiration. The first step of this is the phosphorylation of glucose by hexokinase to prepare it for breakdown to provide energy. The major reason for the immediate phosphorylation of glucose by a hexokinase is to prevent diffusion out of the cell. Organisms use glucose as a precursor for the synthesis of several important substances. Starch, cellulose, and glycogen ("animal starch"). Some of these polymers like starch or glycogen serve as energy stores while others like cellulose have structural roles. In plants, glucose is a product of photosynthesis. In animals, glucose results from the breakdown of glycogen, a process known as Glycogenolysis. In animals, glucose is synthesized in the liver and kidneys from non- carbohydrate intermediates, such as pyruvate and glycerol, by a process known as Gluconeogenesis. Glucose is produced commercially via the enzymatic hydrolysis of starch. Many crops can be used as the source of starch. Maize, rice, wheat Glucose is classified as a monosaccharide, an aldose, a hexose, and is a reducing sugar. Glucose is synthesized by chlorophyll in plants using carbon dioxide from the air and sunlight as an energy source. Glucose is further converted to starch for storage. Fructose Fructose Fructose is found naturally in fruits and vegetables. Fructose is metabolized differently than glucose and other sugars, doesn’t stimulate insulin and is characteristically low glycemic. For these reasons, it’s often used in low-glycemic food applications 15 to 80% sweeter than sucrose, and tastes particularly sweet when cold or in solution Disaccharides Disaccharides Disaccharides are formed when two monosaccharaides are joined together and a molecule of water is removed. Milk sugar (lactose) is made from glucose and galactose sugar cane sugar (sucrose) is made from glucose and fructose. Maltose is made up of two glucose molecules. The two monosaccharides are bonded via a glycosidic bond Can be degraded to monosaccharides by hydrolysis producing water. Sucrose Sucrose • Formed of Glucose and fructose monomers joined together. • known as table sugar • Commercially obtained from sugar cane or sugar beet • Used pharmaceutically to make syrups Lactose • Lactose is a famous disaccharide, resulting from a galactose joining to glucose. • Milk is the most famous source of Lactose. • Used in infant formulations, and as a diluent in pharmaceuticals Maltose Made up of two glucose molecules. Produced when amylase breaks down starch. Obtained from malt sugar and when glucose is caramelized. Polysaccharides Starch Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. produced by all green plants as an energy store It is the most common carbohydrate in the human diet like potatoes, wheat, maize (corn), rice. Cont, Starch • consists of two types of molecules: the linear and helical amylose and the branched amylopectin. • Glycogen, the glucose store of animals, is a more branched version of amylopectin. Used as an additive for food processing, typically used as thickeners and stabilizers in foods such as puddings, custards, soups, and salad dressings, and to make noodles and pastas. Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Another view of amylose and amylopectin, the two forms of starch. Amylopectin is a highly branched structure, with branches occurring every 12 to 30 residues Glycogen • Glycogen is also known as “animal starch”. • It is stored in muscle and liver tissue • Complete hydrolysis yields glucose • Glycogen is the glucose storage polymer in animals, is similar in structure to amylopectin, but glycogen has more branches • The highly branched structure permits rapid release of glucose from glycogen stores, i.e. in muscle during exercise. The ability to rapidly mobilize glucose is more essential to animals than to plants ASSIGNMENTS Students selected in the previous slide are requested to prepare slides about any of the following topics and delivered before next lecture: 1. Importance of carbohydrates in human life. 2. Carbohydrates and cell membrane. 3. Carbohydrates in plants. 4. Importance of glycogen. 5. Disease related to high glucose level. 6. Disease related to low glucose level. 7. Polysaccharides. 8. Functions of glycoprotein. 9. Carbohydrate and energy. 10. Carbohydrate digestion. Students selected for assignment ابانوب اسحاق عبد المسيح سيحه ابراهيم صداق ابراهيم مؤنس ابراهيم ماجد عبد الغني ابو زيد ابراهيم محمد احمد قاسم احمد السيد احمد الريس احمد جمال احمد يوسف الدراجينى احمد جمال الدين عبد الجواد ابو المجد احمد حسن احمد عبد المقصود احمد حسن علي ابراهيم حميدة احمد حسين احمد رسالن Study Questions: Mention the 4 major biomolecules of life. Mention one classification of carbohydrates giving examples. Explain why sucrose is a non reducing while maltose is a reducing sugar Suggested readings: • Harper’s Biochemistry 26th edition