What is light?
advertisement

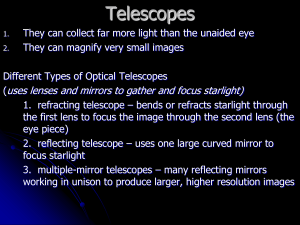
Light and Matter Light in Everyday Life Our goals for learning: • How do we experience light? • How do light and matter interact? The warmth of sunlight tells us that light is a form of energy. Energy is measured in joules. We can measure the flow of energy in light in units of watts: 1 watt = 1 joule/s Interactions of Light 4 process: • Emission • Absorption • Transmission • Transparent objects transmit (allow to pass) light • Opaque objects block (absorb) light • Reflection or Scattering • White light is made up of many different colors Reflection and Scattering Mirror reflects light in a particular direction Movie screen scatters light in all directions Interactions of Light with Matter Interactions between light and matter determine the appearance of everything around us. Objects with color (e.g. a red rose) appear that color because they absorb all the other colors and reflect (or scatter) that one. What is light? • Light is a form of energy that can act either like a wave or like a particle (with energy and momentum) depending on its interaction with matter – A light wave is a vibration of electric and magnetic fields – an electromagnetic wave – Particles of light are bundles (quanta) of energy called photons Waves • A wave is a pattern of motion that can carry energy without carrying matter along with it Leave bobs up and down Properties of Waves Eyes are sensitive to wavelength; we sense wavelength differences as “color”. • Wavelength is the distance between two wave peaks • Frequency is the number of times per second that a wave vibrates up and down (cycles per second or hertz) wave speed = wave length x frequency Mathematically: c = f : the Greek letter lambda Particles of Light • Particles of light are called photons • Each photon has a wavelength and a frequency associated with it • The Energy of a photon depends on its frequency, E = hf • h is a constant of nature called Planck’s constant Wavelength, Frequency, and Energy x f = c = wavelength, f = frequency Visible Light: = 0.5 x 10-6 m, f = 6 x 1014 s-1 (Hz) c = 3.00 x 108 m/s = speed of light E = h x f = photon energy h = 6.626 x 10-34 joule x s = Planck’s constant One photon of visible light carries ~4 x 10-19 joules of energy A 100W bulb emits 2.5 x 1020 photons every second! What have we learned? • What is light? – Light can behave like either a wave or a particle – A light wave is a vibration of electric and magnetic fields – Light waves have a wavelength and a frequency – Photons are particles of light. • What is the electromagnetic spectrum? – Human eyes cannot see most forms of light. – The entire range of wavelengths of light is known as the electromagnetic spectrum. Properties of Matter Our goals for learning: • What is the structure of matter? • What are the phases of matter • How is energy stored in atoms? We need to know this in order to understand the end phase of a star’s life & what’s happening inside a white dwarf or neutron star. What is the structure of matter? Everything is made of atoms Electrons have negative charge and are almost 2000x less massive than protons Electron Cloud 10-15 m Nucleus Atom Neutron: no charge Proton: positive charge Volume of atom = 1,000 trillion times that of nucleus Atomic Terminology • Atomic Number = # of protons in nucleus • Atomic Mass Number = # of protons + neutrons • Molecules: consist of two or more atoms (H2O, CO2) Atomic Terminology • Isotope: same # of protons but different # of neutrons. Example: (4He, 3He) What are the phases of matter? • Phases: – – – – Solid (ice) Liquid (water) Gas (water vapor) Plasma (ionized gas) • Phases of same material behave differently because of differences in chemical bonds. By chemical bonds we mean the electric forces between atoms. Phase Changes Read from bottom to top • Ionization: Stripping of electrons, changing atoms into plasma • Dissociation: Breaking of molecules into atoms • Evaporation: Breaking of flexible chemical bonds, changing liquid into gas • Melting: Breaking of rigid chemical bonds, changing solid into liquid What have we learned? • What is the structure of matter? – Matter is made of atoms, which consist of a nucleus of protons and neutrons surrounded by a cloud of electrons • What are the phases of matter? – Adding heat to a substance changes its phase by breaking chemical bonds. – As temperature rises, a substance transforms from a solid to a liquid to a gas, then the molecules can dissociate into atoms – Stripping of electrons from atoms (ionization) turns the substance into a plasma Learning from Light Our goals for learning: • What are the three basic types of spectra? • How does light tell us what things are made of? • How does light tell us the temperatures of planets and stars? • How do we interpret an actual spectrum? What are the three basic types of spectra? Continuous Spectrum Emission Line Spectrum Absorption Line Spectrum Spectra of astrophysical objects are usually combinations of these three basic types. We can take a picture of a spectrum (lower bar) or we can plot a graph of intensity versus wavelength (upper). Continuous Spectrum Slit in screen • The spectrum of a common (incandescent) light bulb spans all visible wavelengths, without interruption Emission Line Spectrum Each colored “line” is an image of the entrance slit. • A thin or low-density cloud of gas emits light only at specific wavelengths that depend on its composition and temperature, producing a spectrum with bright emission lines Absorption Line Spectrum • A cloud of gas between us and a light bulb can absorb light of specific wavelengths, leaving dark absorption lines in the spectrum Chemical Fingerprints • Electrons in atoms can only occupy certain energy states or levels • The lowest energy state (level 1) is the Ground State • Downward transitions between energy states produce a unique pattern of emission lines (E = hf = hc/) Chemical Fingerprints • Because those atoms can absorb photons with those exact same energies, upward transitions produce a pattern of absorption lines at the same wavelengths Chemical Fingerprints • Each type of atom has a unique spectral fingerprint Chemical Fingerprints • Observing the fingerprints in a spectrum tells us which kinds of atoms are present Energy Levels of Molecules • Molecules have additional energy levels because they can vibrate and rotate • The “spring” just represents the electrical bond between the atoms of the molecule Energy Levels of Molecules • The large numbers of vibrational and rotational energy levels can make the spectra of molecules very complicated • Many of the energy transitions due to vibration and rotation of molecules occur in the infrared part of the spectrum Thermal Radiation • Nearly all large or dense objects emit thermal radiation, including stars, planets, you… • Collisions between atoms in a hot object causes electrons to jump to higher energy levels for a while and then drop down again to emit light. As a result, the photons produced are intimately linked with the temperature (average kinetic energy) in the collisions. Radiation produced this way is called thermal. • An object’s thermal radiation spectrum depends on only one property: its temperature Properties of Thermal Radiation 1. Hotter objects emit more light at all frequencies per unit area. Power per sq. meter = σT4 (Stefan’s Law) 2. Hotter objects emit photons with a higher average energy. maxT ~ 3000 (for in m) (Wien’s Law) Larger objects can emit more total light even if they are cooler. For a sphere (star), luminosity is L = 4πR2σT4 Thought Question Why don’t we glow in the dark? a) People do not emit any kind of light. b) People only emit light that is invisible to our eyes. c) People are too small to emit enough light for us to see. d) People do not contain enough radioactive material. Thought Question Why don’t we glow in the dark? a) People do not emit any kind of light. b) People only emit light that is invisible to our eyes. We glow in the infrared. c) People are too small to emit enough light for us to see. d) People do not contain enough radioactive material. What have we learned? • What are the three basic type of spectra? – Continuous spectrum, emission line spectrum, absorption line spectrum • How does light tell us what things are made of? – Each atom has a unique fingerprint. – We can determine which atoms something is made of by looking for their fingerprints in the spectrum. What have we learned? • How does light tell us the temperatures of planets and stars? – Nearly all large or dense objects emit a continuous spectrum that depends on temperature. – The spectrum of that thermal radiation tells us the object’s temperature. • How do we interpret an actual spectrum? – By carefully studying the features in a spectrum, we can learn a great deal about the object that created it. The Doppler Effect Our goals for learning: • How does light tell us the speed of a distant object? • How does light tell us the rotation rate of an object? How does light tell us the speed of a distant object? The Doppler Effect Waves are compressed in the direction of motion wavelength is decreased frequency is higher Same thing happens for light. Measuring the Shift Stationary Moving Away Away Faster Moving Toward Toward Faster • We generally measure the Doppler Effect from shifts in the wavelengths of spectral lines • The fractional shift is: ( - 0)/0 where 0 is the undisturbed wavelength; this number is equal to the speed of the object relative to that of light (V/c) Doppler shift tells us ONLY about the part of an object’s motion toward or away from us: Pure radial motion – maximum Doppler shift Transverse motion – no Doppler shift Part radial, part transverse – Doppler shift gives Vr = Vcosθ (less than V) θ Vr Thought Question I measure a spectral line in the lab at 500.7 nm. The same line in a star has wavelength 502.8 nm. What can I say about this star? a) It is moving away from me. b) It is moving toward me. c) It has unusually long spectral lines. Thought Question I measure a spectral line in the lab at 500.7 nm. The same line in a star has wavelength 502.8 nm. What can I say about this star? a) It is moving away from me. This is a REDSHIFT b) It is moving toward me. c) It has unusually long spectral lines. And redshift = (502.8 – 500.7)/500.7 = 0.004194 Therefore the radial component of velocity = 0.004194 x c = 1,258 km/s Measuring Redshift How does light tell us the rotation rate of an object? Spectrum of a Rotating Object Slower Faster Spectral lines are wider when an object rotates faster What have we learned? • How does light tell us the speed of a distant object? – The Doppler effect tells us how fast an object is moving toward or away from us. • Blueshift:objects moving toward us • Redshift: objects moving away from us • How does light tell us the rotation rate of an object? – The width of an object’s spectral lines can tell us how fast it is rotating Telescopes: Portals of Discovery A Selection of Major Telescopes Chile 2 x 10m 4 x 8m Mauna Kea, Hawaii Hubble: 2.5m in space X-ray telescope in space New Mexico VLA:27 radio telescopes Eyes and Cameras: Everyday Light Sensors Our goals for learning: • How does your eye form an image? • How do we record images? How does your eye form an image? The lens bends (refracts) light rays and brings to a focus on retina. Refraction • Refraction is the bending of light when it passes from one substance into another • Your eye uses refraction to focus light Focusing Light • Refraction can cause parallel light rays to converge to a focus Image Formation • The focal plane is where light from different directions comes into focus • The image behind a single (convex) lens is actually upside-down! Focusing Light Digital cameras detect light with charge-coupled devices (CCDs) • A camera focuses light like an eye and captures the image with a “detector” • The electronic detectors in digital cameras are similar to those used in modern telescopes What have we learned? • Tell more of the story how digital cameras started in the military and astronomy • How does your eye form an image? – It uses refraction to bend parallel light rays so that they form an image. – The image is in focus if the focal plane is at the retina. • How do we record images? – Cameras focus light like your eye and record the image with a detector. – The detectors (CCDs or charge-coupled devices) in digital cameras are like those used on modern telescopes; these devices convert photons into electrons and then into an electronic image that gets stored in a computer Telescopes: Giant Eyes Our goals for learning: • What are the two most important properties of a telescope? • What are the two basic designs of telescopes? • What do astronomers do with telescopes? What are the two most important properties of a telescope? 1. Light-collecting area: Telescopes with a larger collecting area can gather a greater amount of light in a shorter time. 2. Angular resolution: Telescopes that are larger are capable of taking images with greater detail. Light Collecting Area • A telescope’s diameter tells us its lightcollecting area: Area = π(diameter/2)2 • The largest telescopes currently in use have a diameter of about 10 meters: these are the twin 10m telescopes of the W. M. Keck Observatory which are owned and operated by the California Association for Research in Astronomy (UC and Caltech). Bigger is better Thought Question How does the collecting area of a 10-meter telescope compare with that of a 2-meter telescope? a) It’s 5 times greater. b) It’s 10 times greater. c) It’s 25 times greater. Thought Question How does the collecting area of a 10-meter telescope compare with that of a 2-meter telescope? a) It’s 5 times greater. b) It’s 10 times greater. c) It’s 25 times greater. Fives times larger in diameter means 25 times more area. Angular Resolution • The minimum angular separation that the telescope can distinguish. • At a great distance the pair of lights will look like one rather than two; the light will still be visible but we will not be able to distinguish that it is from two sources. Angular Resolution • Ultimate limit to resolution comes from interference of light waves within a telescope. • Larger telescopes are capable of greater resolution because there’s less interference Angular Resolution • The rings in this image of a star come from interference of light wave. Close-up of a star from the Hubble Space Telescope • This limit on angular resolution is known as the diffraction limit • Angular resolution scales as /D = wavelength; D = telescope diameter What are the two basic designs of telescopes? • Refracting telescope: Focuses light with lenses • Reflecting telescope: Focuses light with mirrors Refracting Telescope • Refracting telescopes need to be very long, with large, heavy lenses Largest lens is ~1m diameter Reflecting Telescope • Reflecting telescopes can have much greater diameters; invented by Isaac Newton • Most modern telescopes are reflectors Designs for Reflecting Telescopes Mirrors in Reflecting Telescopes Twin Keck telescopes on Mauna Kea in Hawaii Segmented 10-meter mirror of a Keck telescope Hard to make really big mirrors by grinding glass. The Keck telescopes have 36 hexagonal segments computer-controlled to act like one large curved mirror. What do astronomers do with telescopes? • Imaging: Taking pictures of the sky • Spectroscopy: Breaking light into spectra • Timing: Measuring how light output varies with time Imaging • Astronomical detectors generally record only one color of light at a time • Several images must be combined to make full-color pictures Imaging • Astronomical detectors can record forms of light our eyes can’t see • False Color is sometimes used to represent different energies of non-visible light Spectroscopy Light from only one star enters • A spectrograph separates the Diffraction different grating breaks wavelengths of light into light before they spectrum hit the detector Detector records spectrum Spectroscopy • Graphing relative brightness of light at each wavelength shows the details in a spectrum Timing • A light curve represents a series of brightness measurements made over a period of time What have we learned? • What are the two most important properties of a telescope? – Collecting area determines how much light a telescope can gather – Angular resolution is the minimum angular separation a telescope can distinguish • What are the two basic designs of telescopes? – Refracting telescopes focus light with lenses – Reflecting telescopes focus light with mirrors – The vast majority of professional telescopes are reflectors What have we learned? • What do astronomers do with telescopes? – Imaging – Spectroscopy – Timing Telescopes and the Atmosphere Our goals for learning: • How does Earth’s atmosphere affect ground-based observations? • Why do we put telescopes into space? How does Earth’s atmosphere affect ground-based observations? • The best ground-based sites for astronomical observing are – – – – Calm (not too windy) High (less atmosphere to see through) Dark (far from city lights) Dry (few cloudy nights) Light Pollution • Scattering of human-made light in the atmosphere is a growing problem for astronomy Twinkling and Turbulence Star viewed with groundbased telescope Same star viewed with Hubble Space Telescope Turbulent air flow in Earth’s atmosphere distorts our view, causing stars to twinkle and images to blur Adaptive Optics A new technology that corrects for atmospheric turbulence Without adaptive optics With adaptive optics How is it done? Rapidly changing the shape of a telescope’s mirror can compensate for some of the effects of turbulence Calm, High, Dark, Dry • The best observing sites are atop remote mountains This is where many astronomers work. Summit of Mauna Kea, Hawaii Why do we put telescopes into space? Transmission in Atmosphere • Only radio and visible electromagnetic waves pass easily through Earth’s atmosphere • We need telescopes in space to observe other forms What have learned? • How does Earth’s atmosphere affect groundbased observations? – Telescope sites are chosen to minimize the problems of light pollution, atmospheric turbulence, and bad weather. • Why do we put telescopes into space? – Forms of light other than radio and visible do not pass through Earth’s atmosphere. – Also, much sharper images are possible because there is no turbulence. Telescopes and Technology Our goals for learning: • How can we observe non-visible light? • How can multiple telescopes work together? • How adaptive optics work animation How can we observe non-visible light? • A standard satellite dish is essentially a telescope for observing radio waves Radio Telescopes • A radio telescope is like a giant mirror that reflects radio waves to a focus Arecibo: 300m Infrared Telescopes SOFIA Spitzer • Infrared (and ultraviolet-light) telescopes operate like visible-light telescopes but need to be above atmosphere to see all IR (and UV wavelengths); examples of a UV telescope – Hubble, GALEX X-Ray Telescopes • X-ray telescopes also need to be above the atmosphere Chandra X-rays are harder to focus … X-Ray Telescopes • Focusing of X-rays requires special mirrors • Mirrors are arranged to focus X-ray photons through grazing bounces off the surface Gamma Ray Telescopes • Gamma ray telescopes also need to be in space • Focusing gamma rays is extremely difficult Compton Observatory Current Missions: SWIFT, FERMI How can multiple telescopes work together? Interferometry • Interferometry is a technique for linking two or more telescopes so that they have the angular resolution of a single large one Interferometry • Easiest to do with radio telescopes • Now becoming possible with infrared and visible-light telescopes also Very Large Array (VLA), Socorro, New Mexico Concept for a 30-meter Giant Segmented Mirror Telescope