Unit 01 Measurement
advertisement

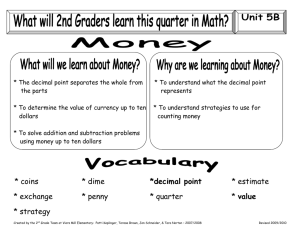
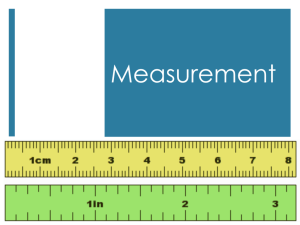
The Beginning Identification/usage of glassware and equipment When diluting acids ALWAYS add the acid to the water to prevent spattering due to heat build up in the solution. When transferring solids use a spatula/scoopula and NEVER use your hands. To prevent spattering when transferring liquids one may use a glass rod near the mouth of the reagent bottle. NEVER heat a stoppered piece of glassware! Define the term meniscus: Curve that forms at the top of a liquid in a glass container due to adhesion of particles to the container. Determining percentage error in an experiment: %error = (measured value - accepted value) accepted value x100% Example: Determine the percent error in an experiment in which the experimental value for volume is 21.20mL and the accepted value is 22.4mL. Table T has various formulas. What is Scientific Notation? number expressed as the product of two (2) factors: 1st: a number from 1 – 9 2nd: a power of 10 Why would we put numbers into Scientific Notation? To simplify larger numbers or very small numbers Convert the following into scientific notation: 602000000000000000000000 .000475 When using instruments with indicated markings, all indicated values are certain. An estimated value between two markings is said to be uncertain. include ALL certain digits and one uncertain digit, no more no less! Significant Example: figures: Which numbers are certain in 7.15cm? How many significant figures does it contain? All NON-ZERO numbers are significant Zero it is significant if: is between two (2) non-zero numbers [2007] whenever you see a decimal point in a number, find the first number other than zero and count [2.00] Zero is NOT significant if: it serves only to hold the place of a decimal [0.239 or 20770] NOTE: if a decimal point follows, a zero becomes significant [20770.] The answer is rounded to be no more precise than the least precise measurement. Look at the decimal place it goes to. Example: Add the following and round to the correct degree of significance. 32.34g + 2.6g + 1.3412g Example: 86.3mL Subtract 531.46mL – Answers should contain no more (no less) significant figures than the least precise measurement. Example: Multiply 24.24cm x 43.9cm Example: Divide 5.1g/213L What is a unit? An indicator for the form of measurement used [cm, L] Note: Selected units in chemistry can be found on Table D Why is it necessary to use units in any scientific measurement? To compare measurements to make them make sense Example: How tall are you? [6 what? Eggs, meters, pounds?] Examples of units in Tools needed for measure: 4g mass 75mL volume 15cm length measure: Balance Graduated cylinder Ruler Table D Prefix Factor Decimal Places Symbol kilo 103 3 k m,L,g centi 100 10-1 0 -1 m,L,g c milli 10-3 -3 m micro 10-6 -6 nano 10-9 -9 n pico 10-12 -12 p Note the appropriate factor and number of places that you must shift the decimal point when converting: (make an L or a 7) moving the decimal the total number of places (ignoring sign). For Example: to convert from nanograms to kilograms you will move 12 spaces to the left (9+3=12). Now using the above table and "sliding decimal rule", perform the following conversions: 1) 460 g= __________mg 6) 1.30 L= _________mL 2) 312 cm = __________m 7) 3.02 g= __________kg 3) 1002 mL= __________L 8) 0.013 g= __________g 4) 100 kg= ___________g 9) 0.027 cm= ________km 5) 8.23 m= __________mm 10) 560 g= __________kg Precision: The reproducibility and the reliability of data during multiple trials of any one experiment. Accuracy: The nearness to the correct or accepted measurement. Measured lab data should be accurate and precise Measurements that are showing precision in data but are not accurate could indicate problems with instruments, contaminated materials etc. The reproducible results indicate a careful worker.