Bonding ppt
advertisement

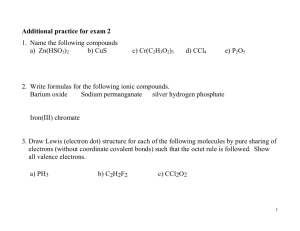
Bonding – Relationships between Microscopic Structure and Macroscopic Properties Chemical Bonds Definition: Any of several forces, especially ionic, metallic and covalent bonds, by which atoms or ions are bound in a molecule or crystal. Chemical Bond and Valence Electron The electrons responsible for the chemical properties of atoms are those in the outer energy level: VALENCE ELECTRONS. – Valence electrons - The electrons in the outer energy level. – Inner electrons -those in the energy levels below. Keeping Track of Electrons Atoms in the same column a. Have the same outer electron configuration. b. Have the same valence electrons. c. Easily found by looking up the group number on the periodic table. d. Group 2A: Be, Mg, Ca, etc. 2 valence electrons Electron Dot Diagrams: Lewis Structures 1. A way of keeping track of valence electrons. 2. How to write them 3. Write the symbol. 4. Put one dot for each valence electron 5. Don’t pair up until they have to X The Electron Dot diagram for Nitrogen Nitrogen has 5 valence electrons. First we write the symbol. Then add 1 electron at a time to each side. Until they are forced to pair up. N Write the electron dot diagram for Na Mg C O F Ne He Ionic Compounds - Ionic Bonds Electron Configurations for Cations 1. Metals lose electrons to attain noble gas configuration. 2. They make positive ions. 3. If we look at electron configuration it makes sense. Na 1s22s22p63s1: 1 valence electron Na+ 1s22s22p6 : noble gas configuration Electron Dots For Cations Metals will have few valence electrons Ca Electron Dots For Cations Metals will have few valence electrons These will come off Ca Electron Dots For Cations Metals will have few valence electrons These will come off Forming positive ions +2 Ca Write the electron configuration diagram and orbital notation for Na Mg C O F Ne He Electron Configurations for Anions 1. Nonmetals gain electrons to attain noble gas configuration. 2. They make negative ions. 3. If we look at electron configuration it makes sense. S 1s22s22p63s23p4: 6 valence electrons S-2 1s22s22p63s23p6: noble gas configuration. Electron Dots For Anions Nonmetals will have many valence .electrons. They will gain electrons to fill outer shell. P -3 P Stable Electron Configuration 1.All atoms react to achieve noble gas configuration. 2.Noble gases have 2 s and 6 p electrons. 3. 8 valence electrons . 4. Also called the octet rule. Ar Ionic Bonding A. Anions and cations are held together by opposite charges. B. Ionic compounds are called salts. C. Simplest ratio is called the formula unit. D.The bond is formed through the transfer of electrons. E. Electrons are transferred to achieve noble gas configuration. Ionic Bonding Na Cl Ionic Bonding: Lewis Structure + Na Cl - Ionic Bonding All the electrons must be accounted for! Ca P Ionic Bonding Ca P Ionic Bonding +2 Ca P Ionic Bonding +2 Ca Ca P Ionic Bonding +2 Ca Ca P -3 Ionic Bonding +2 Ca P Ca P -3 Ionic Bonding +2 Ca P +2 Ca P -3 Ionic Bonding Ca +2 Ca P +2 Ca P -3 Ionic Bonding Ca +2 Ca P +2 Ca P -3 Ionic Bonding +2 Ca +2 Ca +2 Ca P P -3 -3 Ionic Bonding Ca3P2 Formula Unit I. Properties of Ionic Compounds a. Crystalline structure. b. A regular repeating arrangement of ions in the solid. c. Structure is rigid. Ionic Compounds NaCl: Ionic compounds consist of a lattice of positive and negative ions. Lattice: three dimensional array of ions Crystalline Structure 1. forms a lattice crystal- a 3-d geometric structure. a. each negative ion is surrounded by positive ions. b. Lattice energy- the energy required to break one mole of ions from the ionic bond. i. the more – the harder it is to break Ionic solids are brittle + + - + + + + - + + Ionic solids are brittle Strong Repulsion breaks crystal apart. - + - + + - + - + - + Conductivity 1.Conducting electricity is allowing charges to move. 2.In a solid, the ions are locked in place. 3. Ionic solids are insulators. 4. When melted, the ions can move around. 5. Melted ionic compounds conduct. 6. First get them to 800ºC. 7. ELECTROLYTE-Dissolved in water they conduct. Salt water vs salt demo Properties of Ionic Compounds Ions are strongly bonded- because of strong forces between ions they have High melting points. High boiling points. High hardness scale. Very rigid. Very brittle. Do not conduct electricity in solids. Only conduct electricity in melts or aqueous (water) solutions. Do not conduct heat well. Metals - Metallic Bonds Metallic Bonds How atoms are held together in the solid. Metals hold onto there valence electrons very weakly. Think of them as positive ions floating in a sea of electrons. Sea of Electrons Electrons are free to move through the solid. Metals conduct electricity. + + + + + + + + + + + + Malleable Hammered into shape (bend). Ductile - drawn into wires. + + + + + + + + + + + + Malleable Electrons allow atoms to slide by. + + + + + + + + + + + + Properties of Metals Metals are strongly bonded- because of strong forces between positive ions and the “sea” of free electrons surrounding them. High melting points. High boiling point. High hardness scale. Malleable. Ductile. Conduct electricity. Conduct heat well. Molecular Compounds - Covalent Bonds Covalent Bonds How atoms are held together in molecular compounds. Nonmetal elements hold onto valence electrons tightly. All want to gain valence electrons to achieve octet or [He] configuration. The lack of valence electron donor (e.g. metals) results in the sharing of valence electrons. Covalent Bonding H and H H H Q: Who’s going to gain? Who’s going to lose? H H They share them! Covalent Bond, strong Covalent Bonding H H Weak interactions between. Strong interactions within. Properties of Molecular Compounds Molecules interact weakly due to lack of strong forces between them. Low melting points. Many molecular compounds are liquids at room temperature. Low boiling points. Lots of molecular compounds are gases at room temperature. Soft: very low hardness scale. Solids are brittle. Do not conduct electricity in solids nor in aqueous (water) solutions. Do not conduct heat well.