MICR 454L
Emerging and Re-Emerging
Infectious Diseases
Lecture 11:
SARS, Hantavirus
(Reading: Emerging Viruses)
Dr. Nancy McQueen & Dr. Edith Porter
Overview
SARS
Hantavirus
Brief history
Morphology
Genome
Replication cycle
Diseases
Pathogenesis
Diagnosis
Treatment
Prevention
Threat
SARS
SARS Brief History
2003 - an epidemic of severe and often fatal pneumonia broke
out in Southeast China, Hong Kong, and Vietnam.
The disease spread to Toronto, Canada.
Air travel by infected individuals quickly spread the disease to
32 countries resulting in the first pandemic in the twenty-first
century.
Over a 6 month period there were 8,000 cases and 800
deaths
The disease was named severe acute respiratory syndrome
(SARS).
The causative agent was quickly identified as a previously
uncharacterized coronavirus
SARS
Family Coronaviridae
Coronaviruses - divided into 3 groups (I,II, and III) based on
antigenic and genomic sequences
SARS does not fit into any groups
? recombination between an ancestral group II mammalian and a
group III avian virus.
Humans - from contact with palm civets (a cat-like mammal
related to the mongoose)
Probably acquired during slaughter of the civet rather than from
eating the civet.
Palm civets - not the natural reservoir, but rather an intermediate
or amplifier host
Chinese horseshoe bats are the natural reservoir
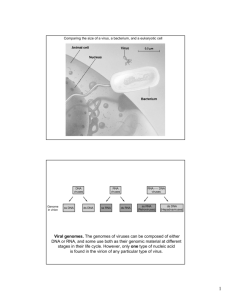
Spherical, enveloped with glycoprotein spikes
Linear SS + RNA genome
Civet
Replication cycle of SARS
Fusion at the
plasma
membrane
Direct translation
of genomic RNA
mRNA
synthesis and
genome
replication in
cytoplasm
Budding into
intracytoplasmic
vesicles
SARS Disease
Transmission
From contact with infected civet
Human to human transmission
•
Through eyes, nose , and mouth
•
•
•
•
Direct contact
Fecal-oral
Droplets produced by coughing and sneezing
? Contact with blood
Mean incubation period: 6.4 days
Symptoms
Fever, dry cough, dyspnea (shortness of breath),
headache,hypoxemia (low blood oxygen concentration)
Other general influenza-like symptoms, including chills, malaise,
loss of appetite, and myalgia.
Gastrointestinal symptoms (less common), including diarrhea
(27%), vomiting (14%), and abdominal pain (13%).
SARS Pathogenesis
Virus initially infects ciliated epithelial cells
Next infects macrophages, and T lymphocytes
Immune cells bring the virus to pneumocytes and surface
enterocytes of the small intestine as well as other organs
The typical clinical course:
improvement in symptoms during the first week of infection
worsening of symptoms during the second week.
may be due to combined effects of patient's immune responses
(proinflammatory cytokines) and uncontrolled viral replication.
Death may result from progressive respiratory failure due to
diffuse alveolar damage (DAD).
Pathologic lesions show inflammatory exudation in the
alveoli and interstitial tissue with hyperplasia of fibrous
tissue and fibrosis.
Fatality rate is 13.2% for patients younger than 60 years and
43.3% for patients aged 60 years or older.
Proposed Role of Immune Cells in SARS Pathogenesis
Dissemination to other organs
SARS-CoV
Infection of epithelial
cells of respiratory tract
Injury to respiratory tract
Infection of immune cells
(Transient)
Immunosuppression
ARDS
SARS
Lung
Pathology
Normal lung tissue
Diffuse alveolar damage pattern of lung injury in SARS patients. (a) Early exudative phase diffuse alveolar
damage showing vascular congestion, with interstitial and airspace edema and inflammatory cell infiltrates
(H&E, original magnification 200); (b) the same field showing fibrinous exudates by Martius scarlet blue stain
(original magnification 200); (c, d) exudative phase diffuse alveolar damage, with hyaline membranes (c,
H&E, original magnification 200; d, elastic trichrome, original magnification 200); (e, f) organizing phase
diffuse alveolar damage (e, H&E, original magnification 100; f, elastic trichrome, original magnification 100).
http://www.nature.com/modpathol/journal/v18/n1/images/3800247f1.jpg
SARS Laboratory Findings
Lymphopenia - due to infection and
destruction of T cells
Extent of decrease correlates with severity of
disease
Mildly elevated aminotransferase indicating
liver damage
Histopathological changes in many organs
SARS Diagnosis
Diagnostic tests for coronavirus infection fall into
two types:
Serological testing
indirect fluorescent antibody testing
ELISA
Molecular testing
RT-PCR
SARS Treatment
Antiviral drugs such as Ribavirin and
interferon have been used
There is no agreement that these antiviral drugs
have been successful in treating SARS or any
coronavirus infection.
Some studies suggest that these treatments
cause more harm than good for the patient.
SARS Prevention
Currently there is no vaccine, but several
different vaccines are under development
Isolation of infected individuals
Hospital personnel must wear masks
Wash hands
Decontaminate all infectious wastes
SARS Threats
No current threats
However, because coronaviruses can
undergo high rates of recombination, it is
feared that other coronaviruses might cross
the species barrier as a result of generation
of recombinants containing both animal and
human coronavirus genes.
~5% of bats in the U.S. carry coronaviruses
Take Home Message SARS
SARS is a coronavirus which is enveloped
and contains a SS +RNA genome
The natural host for SARS is a bat and the
disease was originally transmitted to humans
from infected civets
SARS is characterized by a fever, hypoxemia
and a high mortality rate from respiratory
failure.
Currently no effective treatment or vaccines
Hantavirus
Hantavirus: Brief History (1)
In 1993 in the four corners area of the United States, 24
cases of a severe influenza-like respiratory illness
complicated by respiratory failure occurred in previously
healthy young adults.
Death occurred in 50-60% of the cases.
A hantavirus was ultimately identified as the causative
agent and transmission found to be from contact with
infected rodents (deer mice) or their droppings.
Why did this happen in the Four Corners area? Simply
because there was a "bumper crop" of rodents there,
due to heavy rains during the spring of 1993, which
produced an extra-plentiful supply of the foods that
rodents eat.
Hantavirus Brief History (2)
A new type of hantavirus disease.
Previously, hantavirus infections had been
associated with hemorrhagic fevers, not respiratory
disease.
Documented as early as 1,000 years ago in China.
Since the initial outbreak in the Four Corners
Region, the disease has been confirmed in over half
of the states in the US, with a total of nearly 500
people infected.
Hantavirus Classification
Hantavirus belongs to the family Bunyaviridae,
genus Hantavirus
Hantavirus genus contains several viruses that infect
humans
Some cause a severe hemorrhagic fever with renal syndrome
(HFRS)
Some cause hantavirus pulmonary syndrome (HPS)
All are zoonotic viruses of wild rodents
Spherical, enveloped virus
Linear SS, segmented, - RNA genome
3 segments - small, medium, and large
Hantavirus Replication Cycle
Enter via receptor
mediated
endocytosis
Fusion with endosomal
membrane - uncoating
mRNA synthesis and
genome replication occur
in cytoplasm
Budding into the
Golgi complex
Representative
Pathogenic Hantaviruses
Virus
Disease
Distribution
Mortality rate (%)
Hantaan virus
HFRS (severe)
Asia
5-10
Dobrava virus
HFRS (severe)
Europe (Balkans)
5-10
Seoul virus
HFRS (moderate)
Southeast Asia,
Worldwide
1-2
Puumala virus
HFRS (mild)
North and Central
Europe
<1
Sin Nombre virus
HPS (severe)
North America
>40
Bayou virus
HPS (renal variant)
North America
(US)
>40
Andes virus
HPS (severe)
South America
(Argentina, Chile)
>40
HFRS Disease/Pathogenesis
Transmission via aerosols of viruses from rodent
saliva, urine or feces
Disease divided into five phases
Acute febrile - fever, chills, headache, anorexia, vomiting,
backache
thrombocytopenia with hemorrhagic manifestations;
Hypotensive
kidney edema, proteinuria, renal failure;
cardiovascular instability and shock
Death
Oliguric
Diuretic - suggests improvement
Convalescent - may require 4 months
Viral antigens are detected in brain, spleen, kidneys
and liver.
HPS Transmission to humans
Aerosols of viruses from rodent saliva
Rodent bites (rare)
Oral after touching or eating something that has
been contaminated with rodent urine, droppings, or
saliva
Human-to-human transmission via aerosols
HPS Disease/Pathogenesis
Disease divided into four phases:
Prodromal - non-specific symptoms such as fatigue, fever,
chills, myalgias
Cardiopulmonary - abrupt onset of respiratory failure
which may proceed rapidly and lead to shock, noncardiogenic pulmonary edema and hypotension.
Symptoms include coughing and shortness of breath, with
the sensation of, as one survivor put it, a "...tight band
around my chest and a pillow over my face" as the lungs fill
with fluid.
50% die in 24-48 hours
Diuretic - coincides with rapid clinical improvement
Convalescence - may last several months
HPS Disease progression
Hantavirus Pathophysiology
Hantaviruses preferentially infect endothelial cells
Viruses target the pulmonary capillary walls (HPS) or the
capillary walls in the kidney (HFRS) initiating a cascade of
events culminating in a massive, pulmonary or kidneyspecific inflammatory response
endothelial
damage and edema (TNF and IL-1)
The damage to pulmonary/kidney microvascular
endothelium increases capillary permeability and leads to
even more fulminant pulmonary/kidney edema.
In HFRS - DIC, and hemorrhagic manifestations may follow
Hantavirus Diagnosis
Serology
ELISA
Western immunoblot
Immunohistochemistry
RT-PCR
Hantavirus Treatment
Ribavirin may reduce mortality if given early
in disease - May increase error rate of the
RNA polymerase
Attentive and supportive therapy
HPS - if infected individuals are recognized
early and receive medical care in an intensive
care unit, they may do better.
In intensive care, patients are intubated and given
oxygen therapy to help them through the period of
severe respiratory distress.
Hantavirus Prevention
Eliminate or minimize contact with rodents
Vaccines are in development
Hantavirus Threats
60,000 to 150,000 are hospitalized/year due to HPS or
HFRS
Genomic reassortment by RNA viruses with segmented
genomes is well documented and has the potential to
produce viruses with altered biological activity, host
range, and disease potential.
Genomic reassortment among hantaviruses is known to
occur in nature, but the precise role of genomic
reassortment in the epidemiology of hantavirus
infections is unknown.
HPS causing hantviruses have potential use as
biological weapons
Aerosol infection
Highly lethal
Take Home Message Hantavirus
Hantaviruses are enveloped and contain a
segmented SS - RNA genome.
The natural host for hantaviruses is rodents and
man usually acquires the disease via inhalation after
contact with infected rodents or their droppings.
Hantaviruses cause two different types of disease -
Hemorrhagic fever with renal syndrome (HFRS) characterized by
fever, renal failure, hemorrhaging and shock with a 5-10%
mortality rate
Hantavirus pulmonary syndrome (HPS) characterized by flu
symptoms, coughing, and shortness of breath as the lungs fill
with fluid. The mortality rate is over 50%.
There is potential for new hantaviruses through shift and
drift
Resources
The Microbial Challenge, by Krasner, ASM Press,
Washington DC, 2002.
Brock Biology of Microorganisms, by Madigan and
Martinko, Pearson Prentice Hall, Upper Saddle River,
NJ, 11th ed, 2006.
Microbiology: An Introduction, by Tortora, Funke and
Case; Pearson Prentice Hall; 9th ed, 2007.
Fundamentals of Molecular Virology, by Nicholas
Acheson; Wiley and Sons; 2007
Human Virology by Collier and Oxford, Oxford University
Press; 2nd edition, 2000.
www.chinadaily.com.cn/english/doc/200406/28/xin_11060128103408705697.jpg
www.stanford.edu/~siegelr/RSA/civet.jpg
Resources
www.math.tu-berlin.de/aktMath/site/pics/SARS-Virus.jpg
www.savi-info.ca/SARS_Virus.JPG
www.cdc.gov
http://www.emedicine.com/emerg/topic861.htm
Samuel, Melanie A. and Michael S. Diamond (2006).
Pathogenesis of West Nile Virus Infection: a Balance between
Virulence, Innate and Adaptive Immunity, and Viral Evasion, J
Virol. 80(19): 9349-9360.
Amy C. Simsa, Susan E. Burkett, Boyd Yount, Raymond J. Pickles
(2008). SARS-CoV replication and pathogenesis in an in vitro model
of the human conducting airway epithelium. Virus Research 133:33–
44
Yong Guo, Christine Korteweg, Michael A. McNutt, Jiang Gu (2008).
Review - Pathogenetic mechanisms of severe acute respiratory
syndrome. Virus Research 133:4–12.
Colleen B. Jonsson, Brook G. Milligan, Jeffrey B. Arterburn (2005)
Potential importance of error catastrophe to the development of
antiviral strategies for hantaviruses. Virus Research 107:195–205