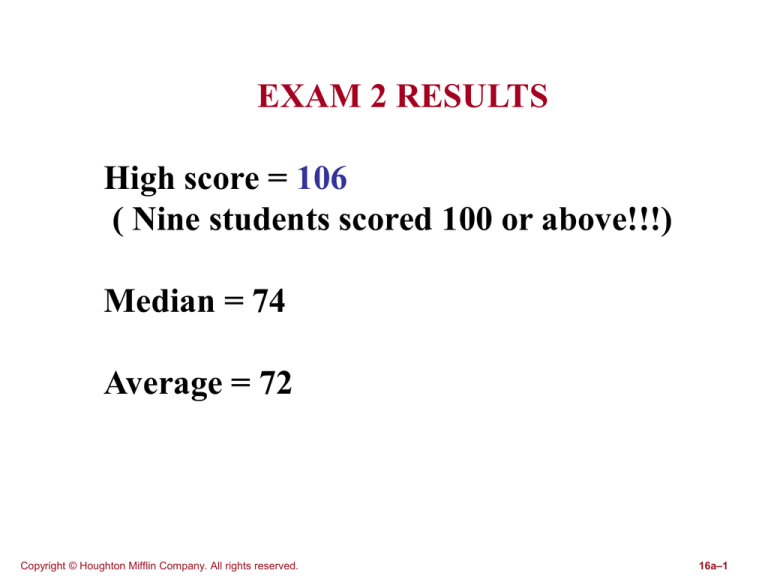
EXAM 2 RESULTS
High score = 106
( Nine students scored 100 or above!!!)
Median = 74
Average = 72
Copyright © Houghton Mifflin Company. All rights reserved.
16a–1
IMPORTANT NOTICE
The third hour exam will be given on
FRIDAY, March 2nd, 2007
Copyright © Houghton Mifflin Company. All rights reserved.
16a–2
More corrections to the text:
Question 15.65: The correct answer is “False”
Question 14.73: Ignore this question; it is not properly formulated.
Thanks to G. Bradds
Copyright © Houghton Mifflin Company. All rights reserved.
16a–3
News from Space
Astronomers have found more than 130 different organic
compounds in outer space (detected from their spectra).
The latest finding is of large amounts of acetone and its close
relative 1,3-dihdroxyacetone (DHA).
O
||
CH3-C-CH3
O
||
CH2OH-C-CH2OH
Acetone
DHA
Acetone is used as a nail polish remover.
DHA is used as an active ingredient in sunless tanning lotion.
Reference: C. Liu, “Cosmic Cosmetics”, Natural History, Feb. 2006, p. 58-59.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–4
Chapter Sixteen
Proteins
About Proteins …
■ Proteins are the most abundant substances in
animal cells (other than water). They account for
almost 50% of a typical cell’s dry mass.
■ The presence of nitrogen sets proteins apart
from lipids and carbohydrates, which contain very
little nitrogen.
■ A typical human cell has roughly 1 million
proteins of about 9000 different varieties. The
human body has about 100,000 different types of
proteins.
■ What is a protein? It is a polymer (a biopolymer) in
which the monomer units are amino acids. Thus,
amino acids are the building blocks of all proteins.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–6
Amino Acids: The Building
Blocks of Proteins
Copyright © Houghton Mifflin Company. All rights reserved.
16a–7
An alpha-amino acid is an organic compound that contains
both an amino group and a carboxylic acid group attached
to the alpha carbon atom.
side chain
R
H
H2N
alpha carbon atom
CO2H
The R group is the side chain which defines the
amino acid.
For example …………..
Copyright © Houghton Mifflin Company. All rights reserved.
16a–8
The 20 Standard Amino Acids, Grouped
According to Side-Chain Polarity.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–9
The 20 Standard Amino Acids, Grouped According
to Side-Chain Polarity. (cont’d)
Copyright © Houghton Mifflin Company. All rights reserved.
16a–10
The 20 Standard Amino Acids, Grouped According
to Side-Chain Polarity. (cont’d)
Copyright © Houghton Mifflin Company. All rights reserved.
16a–11
Protein details …
■ Twenty different “standard amino acids” are found
normally in proteins (see Table 16.1). They are classified
into four groups:
■ Nonpolar amino acids contain a nonpolar side chain; e.g.
Gly, Ala, Val, Leu, Ile, Pro, Phe, and Met.
■ Polar neutral amino acids contain a polar neutral side
chain; e.g. Ser, Cys, Thr, Asn, Gln, Tyr, and Trp.
■ Polar acidic amino acids contain an acidic side chain;
e.g. Asp, Glu. Have a negative charge at physiological pH.
■ Polar basic amino acids contain a basic side chain; e.g.
His, Lys, Arg. Have a positive charge at physiological pH.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–12
Protein Structure and Function: An Overview
■ Proteins are polymers of amino acids.
■ Each amino acid in a protein contains
an amino group, -NH2, a carboxyl group,
-COOH, and an R group, all bonded to
the central carbon atom. The R group
may be a hydrocarbon or it may contain
a functional group.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–13
Amino Acids
20 amino acid types
are used by nature to
build all peptides in
living organisms
presented in the table
Copyright © Houghton Mifflin Company. All rights reserved.
16a–14
■ All but one of the natural amino acids differ
only in the identity of the R group or side chain.
■ The remaining amino acid (proline) is a five-
membered secondary amine.
■ Each amino acid has a three letter shorthand
code--for example, Ala (alanine), Gly (glycine),
Pro (proline).
■ The 20 amino acids present in proteins are
classified as neutral, acidic, or basic depending
on the nature of the side chain.
■ The 15 neutral amino acids are divided into
two groups – polar and nonpolar on the basis of
the nature of their side chain.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–15
■ The sequence of amino acids in a protein and
the chemical nature of their side chains allow
proteins to do their function.
■ Intermolecular forces are of central importance
in determining the shapes and functions of proteins.
■ In biochemistry, noncovalent forces refer to all
interactions other than covalent bonding.
■ The nonpolar side chain of proteins are
described as hydrophobic – they are not attracted
to water.
■ The polar, acidic, and basic side chains are
described as hydrophilic – they are attracted to
water.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–16
Essential amino acids are those which must be obtained
from the diet; i.e. they cannot be biosynthesized by the
human body.
Most animal proteins, such as those obtained from
meat, fish, eggs, and milk contain all of the essential
amino acids. On the other hand, proteins from plants
such as vegetables, grains, and legumes (peas and beans)
lack one or more of the essential amino acids. Therefore,
they must be eaten in complementary pairs to get all of the
essential amino acids in our diet.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–17
The Essential Amino Acids for Humans.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–18
Handedness in Amino Acids
Copyright © Houghton Mifflin Company. All rights reserved.
16a–19
Handedness in Amino Acids
Mirror images of a
hand do not
superimpose on
each other. Image
of left hand on the
mirror looks like the
right hand – objects
that have handedness in this manner
are said to be chiral.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–20
Molecular Handedness and Amino Acids
Amino acids are chiral.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–21
■ Recall: A molecule is a chiral molecule if
four different atoms or groups are attached
to a carbon. The carbon carrying four
different groups is called a chiral carbon.
Chiral molecules have no plane of
symmetry.
■ The two mirror image forms of a chiral
molecule
like
alanine
are
called
enantiomers or optical isomers.
■ Enantiomers have the same connections
but different spatial arrangements of their
atoms.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–22
They’re all lefties!
■ 19 out of 20 natural amino acids are
chiral – they have four different groups
on the a-carbon. Only glycine is achiral.
■ Nature uses only one isomer out of a
pair of enantiomers for each amino acid
to build the proteins.
■ The naturally occurring amino acids
are classified as left-handed or L-amino
acids.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–23
Designation of handedness in amino acid structures
involves aligning the carbon chain vertically and
looking at the position of the -NH2 group.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–24
Rules for designating handedness in amino acids
when drawing their structures are:
1. The CO2H group is put at the top of the structure
and the R group is put at the bottom.
2. The NH2 group is in a horizontal position. When
the NH2 group is on the left, it is the L isomer and
when the NH2 group is on the right, it is the D isomer.
CO2H
H2N
H
R
an L amino acid in a
Fischer projection
structural formula
Copyright © Houghton Mifflin Company. All rights reserved.
16a–25
Acid-Base Properties of Amino
Acids
Copyright © Houghton Mifflin Company. All rights reserved.
16a–26
Acid-Base Properties of Amino Acids
■ Amino acids contain both an acidic
group, -COOH, and a basic group, -NH2.
■ As a result of intramolecular acid-base
reactions, a proton is transferred from the
–COOH group to the –NH2 group
producing a dipolar ion or zwitterion that
has a positive and also a negative charge
and is thus electrically neutral.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–27
H R
H R
OH
H2N
O
O
H3N
O
zwitterion
The amino acid does not exist like this because an
internal acid-base reaction occurs within the molecule.
The NH2 group is a base and it wants to accept a proton,
and the COOH group is an acid and it wants to donate
a proton.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–28
Because they are zwitterions,
amino
acids
have
many
properties that are common for
salts. Such as:
■ amino acids are crystalline
■ amino acids have high
melting points
■ amino acids are somewhat
water soluble.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–29
■ In acidic solutions (low pH), amino acid
zwitterions accept a proton on their –
COO- group to give –COOH and leave
only the positively charged –NH3+ group.
■ In basic solutions (high pH), amino
acid zwitterions lose a proton from their –
NH3+ group to give –NH2 and leave only
the negatively charged –COO- group.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–30
•
•
The charge of an amino acid molecule at any
given moment depends on the identity of the
amino acid and the pH of the medium.
The pH at which the net positive and negative
charges are evenly balanced is the amino
acid’s isoelectric point- the overall charge or
net charge is zero.
H R
H R
H R
OH
H3N
O
low pH (acidic)
Copyright © Houghton Mifflin Company. All rights reserved.
O
H3N
O
neutral solution
O
H2N
O
high pH (basic)
16a–31
For amino acids with acidic or basic side-chains, the
existence of four ionized forms becomes possible.
Consider the amino acid, glutamic acid.
COOH
COOH
H
H
OH
H3N
O
COO
H
O
H3N
O
low pH form
COO
H
O
H3N
O
O
H2N
O
neutral pH form high pH form
moderately low
pH form
Copyright © Houghton Mifflin Company. All rights reserved.
16a–32
More corrections to the text:
Question 13.36: Ignore this question; it is not properly
formulated.
Thanks to A. Dalton
Question 16.29: The answer in the back of the book is
incorrect.
The correct answer is Ser-Ala-Cys.
Thanks to R. Rea
Peptide Formation
Copyright © Houghton Mifflin Company. All rights reserved.
16a–34
■ All amino acids present in a proteins
are -amino acids in which the amino
group is bonded to the carbon next to the
carboxyl group.
■ Two or more amino acids can join
together by forming an amide bond,
which is known as a peptide bond when
they occur in proteins.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–35
■
A dipeptide results when two amino acids combine
together by forming a peptide bond using the amino group
of one amino acid and a carboxyl group of another amino
acid. (This is a condensation reaction; it splits our a water
molecule.)
■
A tripeptide results when three amino acids combine
together by forming two peptide bonds, and so on. Any
number of amino acids can link together and form a linear
chain-like polymer – i.e. a polypeptide.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–36
Naming a dipeptide (or polypeptide):
■ Start from the N-terminal end - that is, the end with the amino
group sticking out. Then start naming the amino acids:
H O H H
| ||
|
|
+
H3N--C---C---N---C---COO|
|
H
CH3
This is
Copyright © Houghton Mifflin Company. All rights reserved.
Gly
Ala
16a–37
Chemical Portraits:
Biochemically Important Small Peptides
Copyright © Houghton Mifflin Company. All rights reserved.
16a–38
Aspartame (Asp-Phe)
• Note first that this is not a “pure” dipeptide, since the carboxyl
group has been esterified with a methyl group.
• Aspartame is 180 times as sweet as sucrose.
• Only the L-L form is sweet; the L-D, D-L, and D-D forms are
bitter.
• Sold as Nutrasweet and Equal. It has no bitter aftertaste,
unlike some other sweeteners..
Copyright © Houghton Mifflin Company. All rights reserved.
16a–39
Levels of Protein Structure
Primary: The sequence of amino acids
Secondary: How the chain is folded in space
(alpha helix, beta pleated sheet)
Tertiary: The folds of the folds; how the
secondary structures are arranged in space
Quaternary: The joining of subunits; many
proteins are clumps of three or four subunits
Copyright © Houghton Mifflin Company. All rights reserved.
16a–40
Primary Structure
of Proteins
The sequence of amino acids in the chain
Copyright © Houghton Mifflin Company. All rights reserved.
16a–41
Insulin
• Insulin is a hormone that regulates blood glucose levels.
• Insulin contains a single strand of 51 amino acids; it’s
primary a.a. sequence was first determined in 1953 by
Frederick Sanger. It took 8 years to work this out, and
Sanger received a Nobel Prize for his work.
• Today amino acid sequences can be relatively easily
determined using automatic machines.
• Understand that many proteins have more than one
strand; the strands are usually linked by disulfide (-S-S-)
bonds.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–42
The primary
structure of human
myoglobin.
Myoglobin is a string
of 153 amino acids.
The actual threedimensional structure
is not shown here.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–43
Secondary Structure
of Proteins
How the chain is configured in space
Copyright © Houghton Mifflin Company. All rights reserved.
16a–44
Four representations of the alpha-helix protein structure.
(a) Arrangement of the protein backbone.
(b) Backbone arrangement with hydrogen-bonding shown.
(c) Backbone atomic detail shown.
(d) Top view of an alpha-helix showing that amino acid side
chains (R groups) point away from the long axis of the
helix.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–45
Two representations of the beta-pleated sheet protein
structure.
(a) A representation emphasizing the hydrogen bonds
between protein chains.
(b) A representation emphasizing the pleats and the
location of the R groups.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–46
The secondary structure of a single protein often
shows areas of -helix and -pleated sheet
configurations, as well as areas of random coiling.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–47
Tertiary Structure
of Proteins
How the secondary structures are
twisted in space
Copyright © Houghton Mifflin Company. All rights reserved.
16a–48
Human insulin, a small two-chain protein, has both
intra-chain and inter-chain disulfide bonds as part
of its tertiary structure.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–49
Types of Noncovalent R group interactions that
contribute to the tertiary structures of proteins:
(a) electrostatic interaction, (b) hydrogen bond,
and (c) hydrophobic interaction.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–50
A schematic diagram showing the tertiary structure of
the single-chain protein myoglobin.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–51
Quarternary Structure of Proteins
Two or more subunits are brought together
to form a complex
Copyright © Houghton Mifflin Company. All rights reserved.
16a–52
A schematic diagram showing the tertiary and
quaternary structure of the oxygen-carrying protein
hemoglobin (four subunits, each with a heme group).
Copyright © Houghton Mifflin Company. All rights reserved.
16a–53
Protein Classification
and Functions
Copyright © Houghton Mifflin Company. All rights reserved.
16a–54
Types of Conjugated Proteins (proteins bonded to
other things)
Proteins can be combined with other types of
compounds, to form heme proteins, lipoproteins,
metalloproteins, etc.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–55
Chemistry
at a
glance:
Protein
Structure
Copyright © Houghton Mifflin Company. All rights reserved.
16a–56
Two Major Protein Types: Fibrous and Globular
Proteins.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–57
Protein Hydrolysis
and Denaturation
Copyright © Houghton Mifflin Company. All rights reserved.
16a–58
Protein denaturation involves loss of the protein’s
three-dimensional structure. Complete loss of such
structure produces a random-coil protein strand.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–59
Selected Physical and Chemical Denaturing Agents
(just examples; not for memorizing)
Copyright © Houghton Mifflin Company. All rights reserved.
16a–60
Enzymes
(Biological Catalysts)
Copyright © Houghton Mifflin Company. All rights reserved.
16a–61
The active site of an
enzyme is usually a
crevice-like region
formed as the result of
the protein’s secondary
and tertiary structural
characteristics.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–62
The lock-and-key model for enzyme activity. Only a
substrate whose shape and chemical nature are
complementary to those of the active site can interact
with the enzyme.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–63
The induced-fit model for enzyme activity. The
enzyme active site, although not exactly
complementary in shape to that of the substrate, is
flexible enough that it can adapt to the shape of the
substrate.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–64
A schematic diagram representing how amino acid R
group interactions can bind a substrate to an enzyme
active site.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–65
Factors That Affect Enzyme
Activity
Copyright © Houghton Mifflin Company. All rights reserved.
16a–66
Effect of temperature
on the rate of an
enzymatic reaction.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–67
Effect of pH on an
enzyme’s activity.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–68
A graph showing how the
reaction rate changes
with change in substrate
concentration at constant
enzyme concentration.
The reaction rate
remains constant above
a certain substrate
concentration because
the enzyme is saturated.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–69
A graph showing the change in reaction rate with
change in enzyme concentration for an enzymatic
reaction. Temperature, pH, and substrate
concentration are constant.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–70
Extremophiles
These are creatures (usually bacteria) that live under
extreme conditions (e.g., high or low temperatures, high or
low pH, or very salty conditions)
■ Often their enzymes have special properties, special
structures. They may resist denaturation at high
temperatures or low pH.
■ Scientists can gather such creatures (for example, from
hot springs in Yellowstone Park) and try to find out how they
can tolerate the extreme conditions.
■ Example: Procter & Gamble wanted an enzyme for its
laundry detergents that could continue to break down grease
at high temperatures.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–71
Turnover Numbers for Selected Enzymes.
Turnover number is the maximum number of substrate
molecules that a single enzyme molecule can cause to
react per second.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–72
Chemistry
at a
glance:
enzyme
activity
Copyright © Houghton Mifflin Company. All rights reserved.
16a–73
Some enzymes are inducible
■ “Inducible” means that cells produce more of the enzyme
when they find more of its substrate present.
■ The point is that it takes energy and effort to make
enzymes, so the body doesn’t want to produce enzymes if
they are not needed.
■ Alcohol hydrogenase is an example.
■ Enzyme induction involves a complex process of
recognition and feedback loops.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–74
Primary Protein Structure
The primary structure of a proteins is its sequence of
amino acids connected by peptide bonds.
The
backbone of a protein is its chain of peptide bonds, and
the amino acid side chains are connected to this
backbone at the carbons.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–75
By
convention,
peptides
and
proteins are always written with the
amino terminal amino acid (Nterminal) on the left and the
carboxyl-terminal amino acid (Cterminal) on the right.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–76
Shape-Determining Interactions in Proteins
■ The essential activity of each protein
depends on its polypeptide chain being held
in a particular shape by the interactions of
the atoms in the side chains.
■ The kinds of interaction that determine the
shapes of protein molecules are shown in
Fig 18.4.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–77
Fig 18.4 Interactions that determine protein shape
Copyright © Houghton Mifflin Company. All rights reserved.
16a–78
Protein shape-determining interactions
are:
•
•
•
•
Hydrogen bonds between neighboring
backbone segments.
Ionic attractions between charged side
chain groups (salt bridges).
Hydrophobic interactions between side
chain groups.
Covalent disulfide (sulfur-sulfur) bonds.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–79
Secondary Protein Structure
• Secondary structure of a protein is the
arrangement of the polypeptide
backbone in space. The secondary
structure includes two kinds of repeating
patterns: -helix and -sheet.
• Hydrogen bonds between backbone
atoms are responsible for both of these
secondary structures.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–80
Alpha-Helix:
A
single protein chain
coiled in a spiral
with a right-handed
(clockwise) twist.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–81
Beta-Sheet: The polypeptide chain is held in place
by hydrogen bonds between pairs of peptide units
along neighboring backbone segments.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–82
Fibrous and Globular proteins
•Fibrous protein: Tough and insoluble protein in
which the chains form long fibers or sheets. The
Secondary structure is responsible for the shape
of fibrous proteins. Wool, hair, and finger nails
are made of fibrous proteins.
•Globular protein: Water soluble proteins whose
chains are folded into compact, globular shape
with hydrophilic groups on the outside.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–83
Tertiary Protein Structure
■ Tertiary Structure of a proteins: The overall three-
dimensional shape that results from the folding of a
protein chain. Tertiary structure depends mainly on
attractions of amino acid side chains that are far
apart along the same backbone. Non-covalent
interactions and disulfide covalent bonds govern
tertiary structure.
■ A protein having the shape its natural shape (as it
would normally appear in an organism) is known as
a native protein.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–84
Quaternary Protein Structure
Quaternary protein structure: The way in
which two or more polypeptide sub-units
associate to form a single three-dimensional
protein unit.
Non-covalent forces are
responsible
for
quaternary
structure
essential to the function of proteins.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–85
Hemoglobin, a protein with
quaternary structure
Copyright © Houghton Mifflin Company. All rights reserved.
16a–86
Chemical Properties of Proteins
Protein hydrolysis: In protein hydrolysis,
peptide bonds are hydrolyzed to yield
amino acids. This is reverse of the
condensation reaction employed in
protein formation.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–87
Protein denaturation: The loss of
secondary, tertiary, or quaternary protein
structure due to disruption of noncovalent interactions and/or disulfide
bonds. (This leaves the peptide bonds
and primary structure intact.)
Copyright © Houghton Mifflin Company. All rights reserved.
16a–88
Agents that cause denaturation
■ Heat The weak side-chain attractions in
globular proteins are easily broken by
heating. Cooking meat converst some of the
insoluble collagens into soluble gelatin.
■ Mechanical agitation Most familiar example
of denaturation of protein by mechanical
agitation is the foaming that occurs during
beating of egg whites.
■ Detergents Very low concentration of
detergents can cause denaturation by
disrupting the association of hydrophobic
side chains.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–89
Agents that cause denaturation (cont.)
■ Organic compounds Polar solvents such
as acetone or ethanol can interfere with
hydrogen bonding by competing for
bonding sites.
■ pH change Excess H+ or OH- ions
reacts with the basic or acidic side chains
in amino acid residues and disrupt salt
bridges.
■ I norganic salts Sufficiently high
concentrations of ions can disrupt salt
bridges.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–90
The active site of an
enzyme is usually a
crevice-like region
formed as the result
of the protein’s
secondary and
tertiary structural
characteristics.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–91
Note on Drug Action
• Most drugs act by blocking enzyme active sites or the
active sites of receptors on cell surfaces.
• Pharmaceutical companies employ teams of scientists
to (a) determine the structures of enzymes and
receptors and (b) design compounds that will fit into
the active sites of a target enzyme or receptor.
• Users of statin drugs (lipitor, zocor, etc., to lower
cholesterol) are advised not to eat grapefruit at the
same time as they take the drugs. Compounds in
grapefruit inhibit an enzyme in the small intestine that
partially breaks down these medications. When this
enzyme is inhibited patients who eat grapefruit get a
larger dose of the drug than intended.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–92
Individual Differences
• Many enzymes differ from one individual to another.
• As a result peoples’ reactions to different drugs and other
substances can differ. Often subgroups in the population
respond differently, making broad studies hard to interpret.
• Example 1: Lactose intolerance. Many individuals lose
their ability to metabolize lactose (milk sugar) as adults, due
to lose of the enzyme lactase.
• Example 2: According to a recent study*, people who
metabolize caffeine slowly are at greater risk of heart
attacks than those who metabolize caffeine quickly. The
difference in metabolism is due to individual variations in the
enzyme Cytochrome P450 (CYP1A2) which metabolizes
caffeine.
* M. C. Cornelis et al., JAMA 295, 1135-1141 (2006)
Copyright © Houghton Mifflin Company. All rights reserved.
16a–93
What you absolutely must understand from Chapter 16.
• Understand that proteins are polymers made from amino acid units.
• Understand the basic structure of an amino acid:
COOH
|
H2N-C-H
|
R
• Appreciate that amino acids are distinguished by their side chains (R).
• Understand that just 20 standard amino acids are typically found in
nature.
• Understand that because four different groups are normally bound to
the central (alpha) carbon atom, amino acids are chiral. Almost all
amino acids found in nature are L-amino acids (H2N- on the left).
Copyright © Houghton Mifflin Company. All rights reserved.
16a–94
What you must know … (cont.)
• Understand that there are four basic types of amino acids:
--Nonpolar amino acids
--Polar neutral amino acids
--Acidic amino acids (have an additional COOH group)
--Basic amino acids (have an additional –NH or –NH2 group)
• You should memorize the structures for six amino acids:
Glycine, Alanine, Proline, Cysteine, Aspartic acid, Lysine
The side chains are R = H, -CH3, a cycle linking the –NH group with
the alpha carbon via 3 –CH2- links, -CH2-COOH, and –(CH2)4-NH2.
• Understand that amino acids take on different ionized or neutral forms
at different pHs. At neutral pH amino acids exist as zwitterions:
H3N+-CH-COONote that both the COOH and
|
the –NH2 group are ionized.
R
Copyright © Houghton Mifflin Company. All rights reserved.
16a–95
What you must know … (cont.)
• Understand that amino acids are joined together by amide
(=peptide) bonds formed in condensation reactions (splitting out
water). The –NH3+ and COO- groups react to form the bond (-NHCO-) and a water molecule (H2O) is lost.
• Appreciate that chains of amino acids are called peptides. The end
with the free –NH3 group is called the N-terminal end, and the end
with the –COO- group is called the C-terminal end.
• The formula for a peptide begins with the N-terminal end, and the
amino acids are listed by their abbreviations, e.g., Gly-Ala-Cys.
• Appreciate that insulin is a hormone with 51 amino acids and that it
was the first protein to have its amino acid sequence determined.
• Know that myoglobin is a protein with 153 amino acid residues. It
has a “heme” group attached to it, and can attach molecular oxygen
and transport it in muscles.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–96
What you must know … (cont.)
• Know the nature of the four levels of protein structure: primary,
secondary, tertiary and quaternary. (Primary = aa sequence.)
• Regarding the secondary structure, understand the natures of the
alpha helix and beta pleated sheet architectures. Understand that
hydrogen bonds hold these structures in place.
• Regarding the tertiary structure, understand that a single protein can
have regions of alpha helix, beta pleated sheets, and random coils. A
single protein can also have two or more strands linked by disulfide (S-S-) bonds (formed when two cysteines react to release H2).
• Appreciate that quaternary structure involves the clumping of two
or more protein subunits through noncovalent attractions. A good
example is hemoglobin, where four subunits come together, each
with a heme group. Hemoglobin carries oxygen in the blood.
• Appreciate that a protein’s structure in space is crucial to its action. A
protein can become denatured, and lose its crucial structure.
Copyright © Houghton Mifflin Company. All rights reserved.
16a–97
What you must know … (cont.)
• Appreciate that there are several ways to classify proteins. One
distinction is between fibrous proteins (mainly used for structure)
and globular proteins (often hormones and enzymes).
• Understand that many proteins are joined in the body with other
substances. Lipoproteins (HDL and LDL) carry cholesterol. Heme
proteins carry oxygen. Glycoproteins (carbohydrates with proteins)
act as antibodies and in other roles.
• Appreciate that many agents, including heat and chemicals, can
denature proteins (disrupt their natural structures).
• Understand that enzymes are biological catalysts. They speed up
reactions without themselves being consumed. They act on
“substrates” (a chemical they affect). The substrate binds to a region
called the “active site” of the enzyme, either in a “lock-key” fit or by
inducing a fit with the enzyme.
• Appreciate that enzymes can speed up biochemical reactions by huge
amounts. (Know turnover number.)
Copyright © Houghton Mifflin Company. All rights reserved.
16a–98
What you must know … (cont.)
• Appreciate how heat can affect an enzyme’s catalytic ability,
and that an enzyme’s action can become saturated at high
substrate concentration.
• Understand that some enzymes are inducible, i.e., cells make
more of these enzymes when their substrates are present.
(Example: alcohol dehydrogenase)
• Understand the types of forces that hold protein structures
together (hydrogen bonds, electrostatic forces, hydrophobic
forces, disulfide bonds).
Copyright © Houghton Mifflin Company. All rights reserved.
16a–99
To Do List
• Read chapter 16!!
• Do additional problems
• Do practice test T/F
• Do practice test MC
• Review Lecture notes for
Chapter Sixteen
Copyright © Houghton Mifflin Company. All rights reserved.
16a–100

