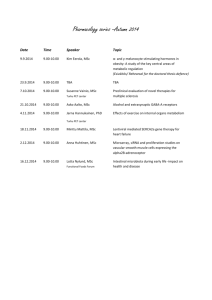
A Chapter 4 Organometallics
advertisement

Chapter 4
Sm
4.1
4.2
4.3
4.4
4.5
4.6.
4.7
Organometallics
The first compounds
Alkyl derivatives
Cyclopentadienyl derivatives
Carbonyl and related complexes
Arene complexes
Cyclooctatetraene derivatives
Divalent lanthanide chemistry
U
U
Sm
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
1
Chapter 4
4.1
Organometallics
The first compounds
cyclopentadienyl Cpcyclooctatetraenyl Cot2-
pentamethylcyclopentadienyl Cp*
Ferrocene:
[Fe(h5-C5H5)2]
Fe
1952
h5 points to the binding
of the 5 carbon atoms
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
2
Chapter 4
M
6L
2a1g
3t1u
2t1u
2t2g
1t1g
Organometallics
1t2u
d-Transition metals:
18-electron rule
points to stable
compounds.
p
s
2eg
d
1t2g
1t1u
1eg
What about f-elements?
Much energy is needed
to “transform” f-elements
into d-elements: cf.
the energies of the
[Xe]4fN-15d16s2 configuration (Ch. 1) versus
[Xe]4fN6s2.
1a1g
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
Slide 24
3
Chapter 4
Organometallics
LnCl3(anh) + 3 NaCp [Ln(Cp)3] + 3 NaCl
(in thf)
J.M. Birmingham, G. Wilkinson,
J. Am. Chem. Soc. 1954,
76, 6210
Ln
Sm
[U(h5-C
U
Cl
5H5)3Cl]
dark red
Ce, Sm: orange
Pr:
pale green
Nd:
pale blue
L. T. Reynolds, G. Wilkinson,
J. Inorg. Nucl. Chem. 1956,
2, 246
UCl4 + 3 NaCp [U(Cp)3Cl] + 3 NaCl
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
(in thf)
4
Chapter 4
U
1968
Organometallics
UCl4 + 2 K2(Cot)
[U(Cot)2] + 4 KCL (in thf)
A. Streitwieser Jr. et al.
J. Am. Chem. Soc. 1968, 90, 7368
d and f organometallic compounds are particularly sensitive
to oxygen and water and this problem is more important
for f-elements in view of their larger ionic radii and their
lower propensity to form covalent bonds.
For f-elements, relativistic effects (see Ch. 1) increase
the complexity of theoretical models aiming at explaining
the chemical bonds in organometallic compounds.
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
5
Chapter 4
4.2
Organometallics
Alkyl derivatives (-bonds)
Most difficult to synthesize. Bulky ligands should be used.
Two main paths:
a) With the help of a bis(trimethylsilyl)methyl ligand
Li[CH(SiMe3)2] + UL3 [U({CH(SiMe3)2}3] (in hexane)
Me
Me
Me
Si
Me
C Si Me
H
L =
O-
Me
C3-symmetry
U-C: 2.48 Å
C-U-C: 107.7o
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
6
Chapter 4
Organometallics
b) Smaller alkyl groups may also be used, often leading to
negatively charged species
LuCl3 + 4 Li(t-but) + Me2N-(CH2)2-NMe2
[Lu(t-but)4]-[Li(tmed)2]+ (in Et2O/pentane)
H. Schumann et al., J. Organomet.
Chem. 1986, 306, 215
CS symmetry
Lu-C
2.32-2.43 Å
C-Lu-C
107-109 o
Allows alkylation reactions:
HO
O
tBut
O
tBut
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
7
Chapter 4
Organometallics
LuCl3 + 6 LiMe + 3 dme [Li(dme)]+3 [Lu(Me)6]3- + 3 LiCl
The compounds are extremely
air- and water-sensitive.
They were obtained with
several Ln.
The structure of [Er(Me)6]3is also known, with
[Li(Me2N(CH2)2NMe2]+ as
counterion
H. Schumann et al., J. Organomet. Chem.
1984, 263, 29.
Lu-C
C-Lu-C
C-Lu-C eq
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
2.48-2.57 Å
86-96 o
176 o
8
Chapter 4
Organometallics
Decomposition of alkyl complexes via b-elimination
Ln
H
H
R
Ln
LnH +
R
R
f-elements do not form strong bonds with alkenes, so
that decomposition proceeds easily
Conditions for b-elimination:
- the b-carbon must bear a H atom
- the Ln-C-C-H fragment must be able to adopt a
planar conformation
- the metal ion must have an appropriate empty
orbital for binding the H-atom
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
9
Chapter 4
Organometallics
f-Elements have a large number of empty orbitals, so
that they are extremely susceptible to b-elimination.
The strategy for producing alkyl derivatives with low
coordination numbers therefore involves bulky ligands for
which b-elimination is not possible, such as CH(SiMe3)2.
Agostic interaction
H
Me
Me
H
C H
Si
Me3Si
U
L
L
U-H-C bridges are formed with
H-atoms in g position with respect
to U: this is called a g-agostic
interaction.
Therefore, the coordination number
is larger than 3 in this compound
and this is often the case for lowCN organometallic compounds
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
10
Chapter 4
4.3
Organometallics
Cyclopentadienyl derivatives (-bonds)
Cp and its derivatives are among the most popular ligands
in organometallic chemistry of d- and f-transition metals.
Cp forms both covalent and ionic complexes with 1-5
C-atoms coordinated. The h5-mode is usually considered
to occupy 3 coordination sites (this is somewhat arbitrary).
Simple LnCp3 cyclopentadienyls
• LnCp3 features polar Ln-Cp bonds as shown by:
2 LnCp3 + 3 FeCl2 3 FeCp2 + 2 LnCl3
• Rapid exchange of Cp ligands occurs
• LnClCp2 compounds can be isolated as dimers or as
Lewis acid adducts
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
11
Chapter 4
Cl
Ln
Ln
Cl
Organometallics
Most dimers
feature Cl- as
bridging ligands
Cl
Cl
Ln
O
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
Ln
N
12
Chapter 4
Organometallics
Structural changes along the series
La-Pr
“11-coordinate” [Ln(h5Cp)3(h2Cp)]
(under the form of a coordination polymer)
Ln
Cp is counted
as tridentate!
Ln
Ln
Y, Sm-Yb
“9-coordinate” [LnCp3]
Sm-C
2.75 Å
G.Laubereau & J.H. Burns Inorg.Chem.
1970, 9, 1091,
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
13
Chapter 4
Sc, Lu
Organometallics
“8-coordinate” [Lu(h5-Cp)2(h1-Cp)2
(coordination polymer)
Ln
Ln
Ln
Simple AnCpn cyclopentadienyls
AnCp3 compounds behave similarly to LnCp3. Most of
the Cp chemistry of Th, U, Pa and Np elements
however involves +4 oxidation state:
AnCl4 + n NaCp [AnCpnCl4-n] + n NaCl
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
14
Chapter 4
[An(Cp)4] are the only complexes
with four h5-Cp coordinated in
a tetrahedral arrangement.
As a comparison, the ZrIV
analogue is [Zr(h5-Cp)3(h1-Cp)]
An
Th-C
Th-Cp
U-Cp
Organometallics
2.87 Å
2.61 Å
2.59 Å
R. Maier e al., J. Alloys & Cmpnds
1993, 190, 269.
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
15
Chapter 4
Organometallics
[UCp3Cl] + FeCl2 ferrocene
indicating a more covalent U-Cp bond than in the
analogue Ln compounds. On the other hand, the Cl
ligand in [AnCp3Cl] can easily be substituted, making
these complexes important synthons in An organometallic chemistry.
Substituted Cp’s are also used, as
in this ThIV derivative:
[Th(Me3SiCp)3Cl]
R. C. Blake et al., J. Organomet.
Chem. 1998, 551, 261.
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
16
Chapter 4
Organometallics
An
AnCl4
TlCp
NaOEt
An
LiMe
An
Cl
OEt
NaBH4
An
CH3
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
BH4
17
Chapter 4
Organometallics
Substituted cyclopentadienyls
Cp
Cp*
Me
Me
Me Me
Me
Si
Me Si
Si Me
Me
Me
Me
Me Si
Si
Me
Me
Me
Me
Cp’’
The substitution gives
more stable, more soluble
compounds which are
easier to crystallize.
However, Ln(Cp*)3 and
An(Cp*)3 are not very
stable, because the
ligand is too bulky.
On the other hand, [Ln(Cp*)2Cl], [An(Cp*)2Cl] and
[An(Cp*)2Cl2] are versatile and have an extensive
chemistry.
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
18
Chapter 4
Organometallics
[Sm(Cp*)3]
Trigonal co-ordination
Sm-C
2.82 Å
Cp*-Sm-Cp*
120o
W. J. Evans et al., J. Am. Chem. Soc.
1991, 113, 7423
2 [Sm(Cp*)2] + Cot [Sm(Cp*)3] + [Sm(Cp*)(Cot)]
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
19
Chapter 4
Organometallics
An active Th catalyst (A) for ethene polymerization:
It also inserts CO:
H
Cl
Th
Th
2
Cl
H
2 LiMe
2 CO
Me
2
O
Th
Me
H2
Th
H
Th
H
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
A
20
Chapter 4
Organometallics
1,2-bis(dimethylphosphinoethane)
Th
H
+ 2 [Et3NH][BPh4]
Th
H
…
KCp*(18C6)
Th-C
Th-H
dmpe
[Th(Cp*)3H]
2.87 Å
2.33 Å
W.J. Evans et al., Organometallics
2001, 20, 5489.
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
21
Chapter 4
Organometallics
tBut
substituents are also commonly grafted onto Cp,
yielding a wealth of interesting compounds
[Yb{(tbut)2Cp}3]
[{YbCl(tButCp)2}2]
A.V. Khvostov et al.
J. Organomet. Chem.
1998, 568, 113.
J.S. Ren et al., Jiegou Huaxue
1997, 16, 380
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
22
Chapter 4
4.4
Organometallics
Carbonyl and related complexes
d-Transition metal chemistry:
M
C
-donation
O
M
C
O
-retrodonation
4f-Transition elements: the filled metal orbitals are not
outside orbitals so that -overlap is not effective.
Ln(g) + n CO (g) Ln(CO)n (g), n = 1-6, in Ar at <40 K
Identified by IR spectra. Decompose upon increasing T.
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
23
Chapter 4
Organometallics
Laser-ablated Gd atoms and Gd2 dimers co-deposited
with CO onto a CsI window (under Ar):
Gd(CO)x
Gd
x = 1-3
Gd
C
O
C
O
Gd
C
Activation of CO
C
O
Gd
Studied by vibrational
spectroscopy with 12C/13C
substitution + DFT calculations
Gd
Gd
Cleavage of CO
O
Xi Jin et al. J. Phys. Chem. A 2006, 12585
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
24
Chapter 4
Organometallics
5f-Transition elements
More covalent so that AnCp3CO complexes are known
Me3Si
SiMe3
U
U
CO
CO
SiMe3
n(CO) = 1976 cm-1
n(CO) = 1900 cm-1
Since n(CO) = 2146 cm-1 for free CO, these values
point to a significant amount of back bonding.
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
25
Chapter 4
Organometallics
Dinitrogen, isocyanide, alkene, and alkyne compounds
Dinitrogen is isoelectronic with CO, but less efficient for
-bond formation.
In the case of f-elements, is forms complexes only
with the low oxidation states, e.g. SmII, YbII, UIII.
R
R
N
N
R
N
U
N
N
N
U
N
R
Isocyanides CN-R:
N
R
N
N
R
[LnCp3(CN-Et)] have n(CN)
values higher than in free CN-R
pointing to negligible backbonding
Alkene and alkyne complexes are also very difficult to
make and only occur with low oxidation states
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
26
Chapter 4
Organometallics
4.5 Arene complexes
h6-arene ligands (such as benzene) are -acceptors and
d-donors.
Note ,,d,and frefer to the symmetry of the bonding
interaction with respect to the metal-ligand axis:
they correspond to 0, 1, 2, and 3 nodal planes.
Arene ligands usually form complexes when the metal is
in a low oxidation state.
tBut
Ln(g) + tBut3Ph
tBut
-196oC
0-valent compounds
sublime in vacuo at
100 oC
tBut
Ln
tBut
tBut
Ln = Pr, Nd,
Gd, Tb, Dy,
Ho, Er, Sc, Y
tBut
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
27
Chapter 4
Organometallics
The 0-valent compounds form only for Ln for which
the promotion energy [Xe]4fN6s2 [Xe]4fN-15d16s2 is
relatively small (cf. Ch. 1).
Therefore, involvement of the 5d electron is holding the
bis(arene) Ln0 compounds together.
A SmIII compound is also known:
SmCl3 + 3 AlCl3 + PhMe6
Cl
Cl
Al
Cl
Cl
Sm
Cl Cl
Cl
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
Cl
Al
Cl
Al
Cl
Cl
Cl
28
Chapter 4
4.6
Organometallics
Cyclooctatetraene complexes
Cot2- is ideally suited for f-element organometallic
chemistry because it has a high valency and is sterically
bulky (as much as Cp*).
Uranocene, D8h symmetry
The two rings are eclipsed in conformation, therefore the
D8h symmetry
The major source of binding is the interaction between
the ring e2g orbitals and the U(6d) orbitals (d-bonds).
The second source of binding is between the ring e2u
orbitals and the U(5f) orbitals (f-bonds).These are only
available with f-elements.
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
29
Chapter 4
Organometallics
d-bonds (major contribution to binding)
6d
Two vertical
nodal planes,
but no nodal
plane between
the rings,
hence e2g
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
30
Chapter 4
Organometallics
f-bonds
Nodal plane
between the
rings, hence
e2u
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
31
Chapter 4
Organometallics
e2g
e1g
antibonding
e2u+e2g
6d
a1g
e2u
a2u
e1u
5f
e3u
e1u+e1g
d
f
a2u+a1g
20 e-
U
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
UIV
2 e32
Chapter 4
Organometallics
Lanthanide Cot2- complexes
[Ce(h8-Cot)2] is known for a long time. Recent calculations
tend to prove that it should however be formulated as
[Ce3+(Cot2)3-]. It can be reduced by potassium:
[Ce(h8-Cot)2] + K K[Ce(h8-Cot)2]
Other Ln compounds are obtained as follows:
LnCl3 + 2 K2Cot K[Ln(h8-Cot)2] + 3 KCl
These compounds are essentially ionic. They react readily
with UCl4 to give uranocene.
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
33
Chapter 4
Organometallics
In sterically crowded complexes such as [Ln(Cp*)3], (Cp*)functions as one-electron reductive species.
[Ln(Cp*)3] 1e- + ½ (Cp*)2 + [Ln(Cp*)2]+
Example: synthesis of the
mixed species [Sm(Cp*)(Cot)]
[Sm(Cp*)3] + Cot [SmCp*Cot] +(Cp*)2
W. J. Evans et al., Dalton Trans. 2000,
1609
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
34
Chapter 4
Organometallics
4.7 Divalent lanthanide chemistry
Initial studies involved SmII, EuII, YbII
SmI2 + 2 NaCp* [Sm(Cp*)2(thf)2] (in thf)
[SmII(Cp*)2(thf)2] [SmII(Cp*)2] + 2 thf (upon heating)
purple
Sm-C:
2.79 Å
2.86 Å
W. J. Evans et al., J. Am. Chem. Soc.
1981, 103, 6507 and 1984, 106, 4270
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
35
Chapter 4
Organometallics
These species are highly reactive and generate interesting
redox chemistry. For instance, they can add dinitrogen
upon crystallization:
2 [Sm(Cp*)2] + N2 [Sm2(Cp*)4N2]
First N22- complex of a
Ln ion
Sm-C
2.73 Å
Sm-N
2.36 Å
N-N
1.09 Å
similar to N2 ?
W. J. Evans et al., J. Am. Chem. Soc.
1988, 110, 6877
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
36
Chapter 4
Organometallics
An O2- bridging ligand: [SmIII(Cp*)2-O-SmIII(Cp*)2
2 [SmII(Cp*)2(thf)2] + N2O [SmIII2(Cp*)4(m-O)] + N2
Sm-O
Sm-C
2.09 Å
2.74 Å
In fact, many O-containing substrates produce this
compound (NO, thf, a.s.o)
W. J. Evans et al., J. Am. Chem. Soc. 1985, 107, 405
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
37
Chapter 4
Organometallics
Insertion of trans-azobenzene: [SmII2(Cp*)4N2Ph2]
2 [SmII(Cp*)2(thf)2] +N2Ph [SmIII2(Cp*)4(m-h1:h1-N2Ph2)]
Magnetic data indicate the
presence of SmIII (1.9 M.B.)
blue
Sm-C
Sm-N
N-N
Sm-H distances (ortho
position) are close to
agostic interaction
2.74 Å
2.40 Å
1.25 Å, identical to neutral N2Ph2
expected distance: 1.44 Å
W. J. Evans et al., J. Am. Chem. Soc. 1988, 110, 4983
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
38
Chapter 4
Organometallics
Insertion of CO:
[SmII2(Cp*)4Phe2N2] + 2 CO
[SmII2(Cp*)4Phe2N2]
(in thf)
green
C2h
W. J. Evans et al., J. Am. Chem. Soc.
1988, 110, 4983
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
39
Chapter 4
Organometallics
Chalcogenolate complexes:
[SmII(Cp*)2(thf)2] + PhEEPh [SmIII2(Cp*)4(PhE)2] + 2 thf
E = S, Se, Te
orange
[SmIII2(Cp*)4(PhS)2]
Sm-S
2.76 Å
W.J. Evans et al., Inorg. Chem. 2005, Published on the web, May 13
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
40
MSc: f-Elements, Prof. J.-C. Bünzli, 2008
41